Method for preparing soda ash by converting sodium sulfate in low-grade rock salt or glauber salt ores
A low-grade, sodium sulfate technology, applied in carbonate preparations, calcium/strontium/barium sulfate, etc., can solve problems such as environmental pollution, distillation waste liquid, waste residue need to be discharged, and calcium chloride market capacity is limited.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
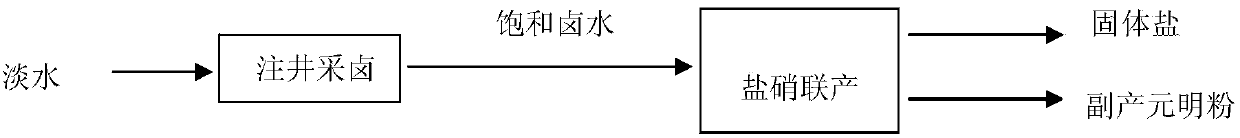
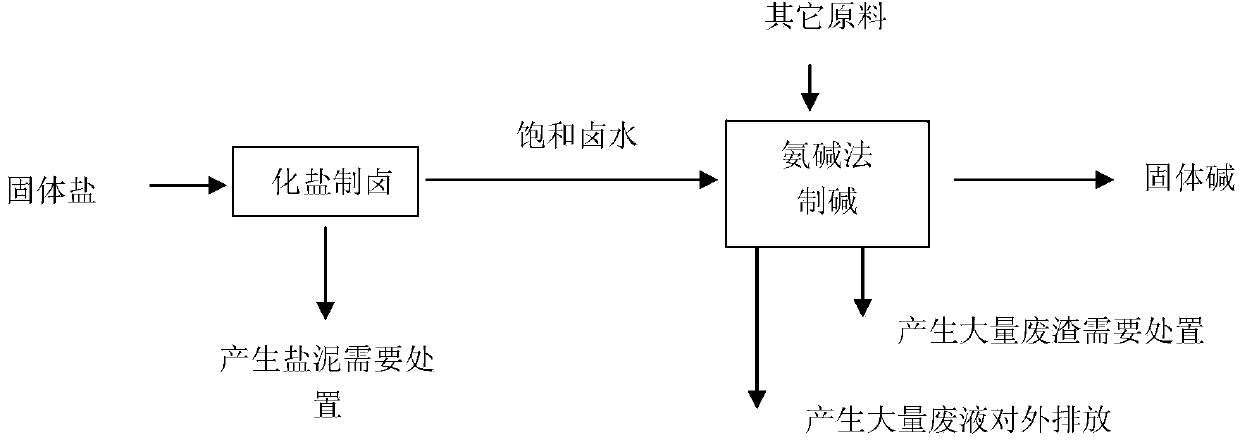
Image
Examples
Embodiment 1
[0094] Example 1: Low-grade rock salt mineral resources, the output of soda ash is 600,000 t / year, and about 3 million m3 of low-nitrate brine is needed 3 / year as raw material, will produce 5.4 million m 3 192,000 tons of soda-making waste liquid and 192,000 tons of soda-making waste residue.
[0095] 5.4 million m 3 Because the content of calcium chloride is higher than that of sodium sulfate, it needs to be diluted with water and then injected into the well to extract brine. The dissolution chamber is utilized in equipment, and calcium chloride and sodium sulfate react and clarify in the dissolution chamber. According to calculations, the amount of water added is 8.43 million cubic meters of fresh water, and 725,000 tons of sodium sulfate in low-grade rock salt resources is converted into sodium chloride, and 695,000 tons of calcium sulfate is generated, which settles to the bottom of the cave in the underground cavern. If the take-bet ratio is 0.9, you will get 12.45 mi...
Embodiment 2
[0098] Example 2: Low-grade Glauber's salt mineral resources 1), the output of soda ash is 600,000 t / year, and about 3 million m3 of low-nitrate brine is required 3 / year as raw material, will produce 5.4 million m 3 192,000 tons of soda-making waste liquid and 192,000 tons of soda-making waste residue.
[0099] 5.4 million m 3 Because the content of calcium chloride is lower than that of sodium sulfate, it needs to be concentrated and injected into the well to extract brine. The dissolution chamber is utilized in equipment, and calcium chloride and sodium sulfate react and clarify in the dissolution chamber. After calculation, it needs to be concentrated to CaCl 2The content is about 140.2g / L, and the concentrated quantity is 4.04 million cubic meters. Among the low-grade rock salt resources, 725,000 tons of sodium sulfate is converted into sodium chloride, and 695,000 tons of calcium sulfate is produced, which settles to the bottom of the cavern. If the take-bet ratio is...
Embodiment 3
[0103] Example 3: Low-grade Glauber's salt mineral resource 2), the output of soda ash is 600,000 t / year, and about 3 million m of low-nitrate brine is needed 3 / year as raw material, will produce 5.4 million m 3 192,000 tons of soda-making waste liquid and 192,000 tons of soda-making waste residue.
[0104] 5.4 million m 3 The alkali-making waste liquid is directly injected into the well to extract brine because the content of calcium chloride is equivalent to the content of sodium sulfate. The dissolution chamber is utilized in equipment, and calcium chloride and sodium sulfate react and clarify in the dissolution chamber. According to calculations, 725,000 tons of sodium sulfate in the low-grade rock salt resources was converted into sodium chloride, and 695,000 tons of calcium sulfate was generated, which settled down to the bottom of the cave in the underground cavern. Take note ratio 0.9, get 4.86 million m 3 Low-nitrate brine, the average sodium sulfate content in t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com