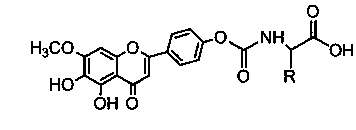
Carbamate derivates of scutellarin and seutellarein, and application thereof
A technology of aglycon carbamate and scutellarin aglycon, which is applied in the field of pharmacy, and can solve the problems of high oral bioavailability, low oral bioavailability, and unsatisfactory drug efficacy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
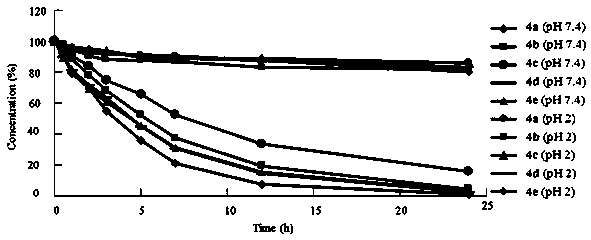
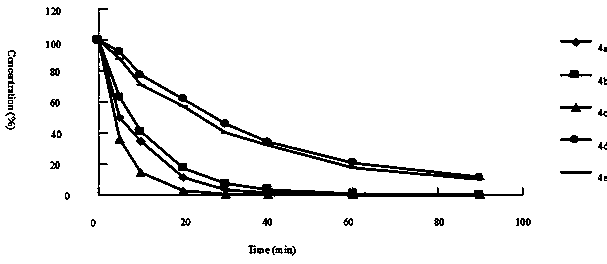
Image
Examples
Embodiment 1
[0109] Add methyl ethyl acetate (770mg, 1.6mmol) to a 100ml dry reaction bottle, 15ml of anhydrous DMF to make it completely dissolved, dissolve tert-butyl-protected glycine isocyanate (381mg2.43mmol) with 10ml of anhydrous THF and add to the reaction system , added triethylamine (34.02μL, 0.243mmol), reacted at 50°C under nitrogen protection for 10 hours, evaporated the solvent under reduced pressure, separated and purified by silica gel column chromatography, and the eluent was chloroform:methanol=15:1 (volume ratio) to obtain 374.6 mg of white-like scutellarin-6″-methyl ester 4′-tert-butyl-protected glycine carbamate solid, with a yield of 37%;
[0110] 1 H-NMR (400MHz, DMSO-d 6 ): δ13.05(brs, 1H), 10.46(brs, 1H), 8.18(t, 1H, J=5.6Hz), 7.97(d, 2H, J=8.8Hz), 7.12(s, 1H), 6.95 (d, 2H, J=8.8Hz), 6.92(s, 1H), 5.53(d, 1H, J=5.6Hz), 5.34(brs, 2H), 5.20(brs, 1H), 4.21(d, 1H, J=9.6Hz), 3.73(d, 2H, J=6.0Hz), 3.65(s, 1H), 1.42(s, 9H).
[0111] The compound (158 mg, 0.24 mmol) obt...
Embodiment 2
[0114] Add methyl ethyl acetate (650mg, 1.37mmol) to a 100mL dry reaction flask, dissolve it completely in 15mL of anhydrous DMF, dissolve tert-butyl-protected alanine isocyanate (351mg, 2.05mmol) in 10mL of anhydrous THF, and add Add triethylamine (28.7μL, 0.205mmol) to the reaction system, react at 50°C for 10 hours under nitrogen protection, evaporate the solvent under reduced pressure, separate and purify by silica gel column chromatography, and the eluent is chloroform:methanol=15:1 (V / V) to obtain 457.8 mg of white-like scutellarin-6″-methyl ester 4′-tert-butyl-protected alanine carbamate solid, with a yield of 51.6%.
[0115] 1 H-NMR (400MHz, DMSO-d 6 ): δ13.05(brs, 1H), 10.46(brs, 1H), 8.25(d, 1H, J=7.2Hz), 7.97(d, 2H, J=8.8Hz), 7.12(s, 1H), 6.95 (d, 2H, J=8.8Hz), 6.90(s, 1H), 5.54(d, 1H, J=4.8Hz), 5.36(brs, 2H), 508(brs, 1H), 4.21(d, 1H, J=9.2Hz), 3.99(m, 1H), 1.40(s, 9H), 1.31(d, 3H, J=7.2Hz).
[0116] The compound (158mg0.24mmol) obtained in the previous step wa...
Embodiment 3
[0119] Add methyl ethyl acetate (680.68mg1.34mmol) to a 100mL dry reaction bottle, 15mL of anhydrous DMF to make it completely dissolved, dissolve tert-butyl-protected valamino acid isocyanate (428mg, 2.15mmol) with 10mL of anhydrous THF and add Add triethylamine (28.0 μL, 0.200 mmol) to the reaction system, react at 50°C for 10 hours under nitrogen protection, evaporate the solvent under reduced pressure, separate and purify by silica gel column chromatography, and the eluent is chloroform:methanol=15:1 (Volume ratio), 398.1 mg of white-like scutellarin-6″-methyl ester 4′-tert-butyl-protected valine carbamate solid was obtained, with a yield of 44%.
[0120] 1 H NMR (400MHz, DMSO-d 6 ): δ13.07(brs, 1H), 10.48(brs, 1H), 8.14(d, 1H, J=8.4Hz), 7.99(d, 2H, J=8.8Hz), 7.13(s, 1H), 6.97 (d, 2H, J=8.8Hz), 6.92(s, 1H), 5, 53(brs, 1H), 5.38(brs, 2H), 512(brs, 1H), 4.21(d, 1H, J=9.2 Hz), 3.87-3.83(m, 1H), 3.67(s, 3H), 2.12-2.07(m, 1H), 1.44(s, 9H), 0.96(d, 6H, J=9.2Hz).
[0121] The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com