Hyperforin analogs, methods of synthesis, and uses thereof
A halogen and compound technology, applied in the field of preparing hyperforin analogues described herein, can solve problems such as difficult synthesis of hyperforin analogues, inapplicability of hyperforin, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0493] In order that the invention described herein may be more fully understood, the following examples are set forth. It should be understood that these examples are for illustrative purposes only and do not limit the invention in any way.
[0494] Synthesis of Hypericin
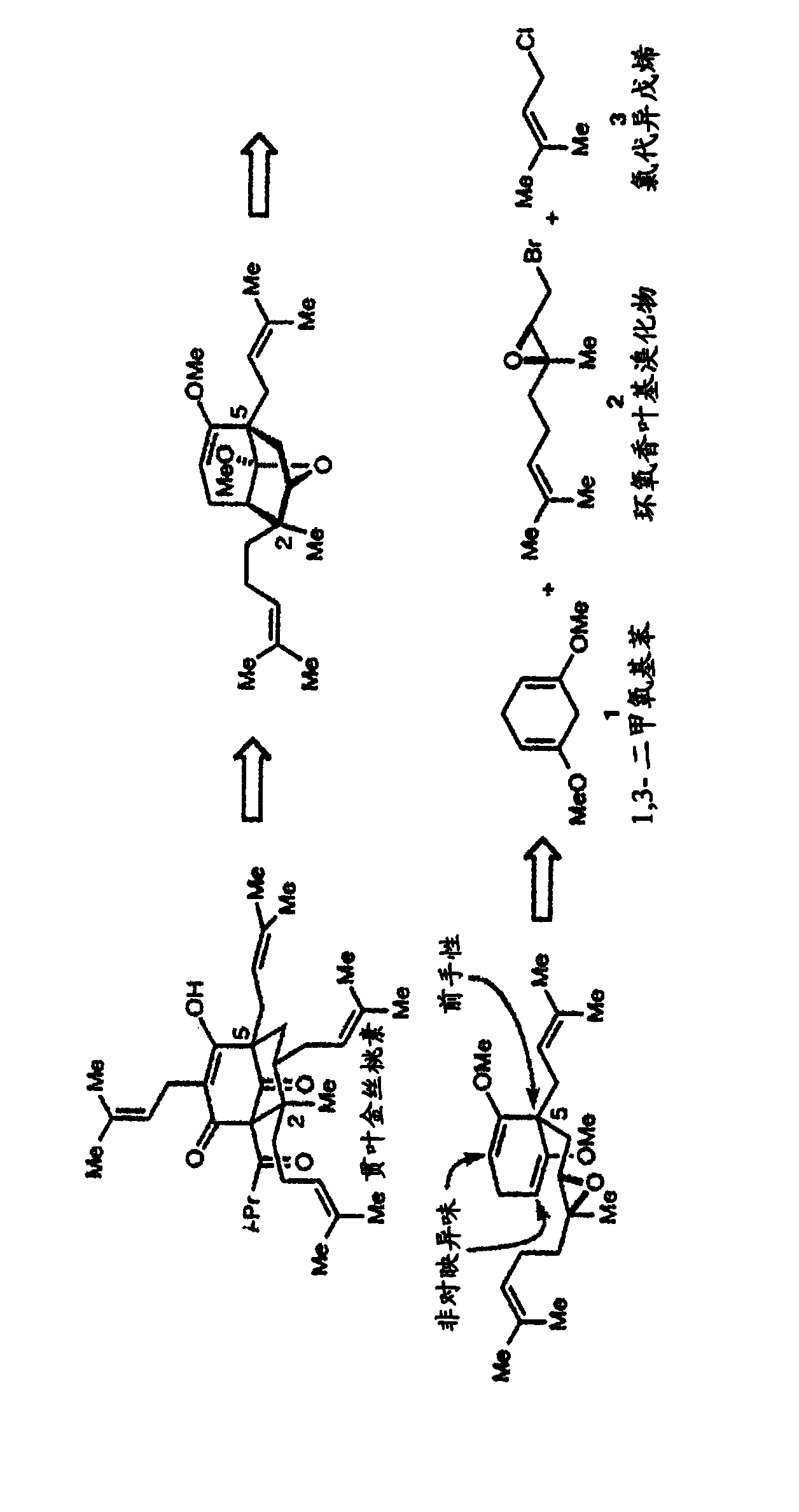
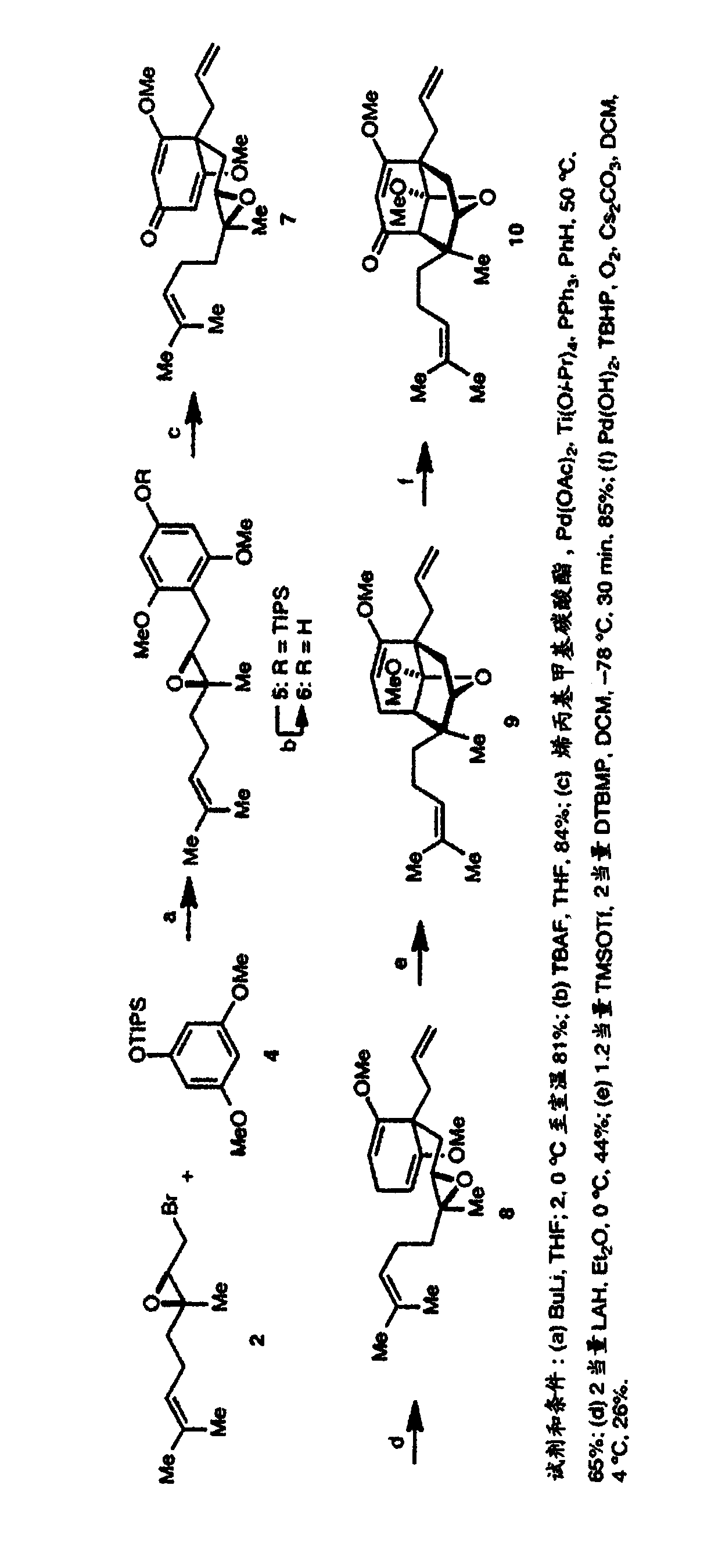
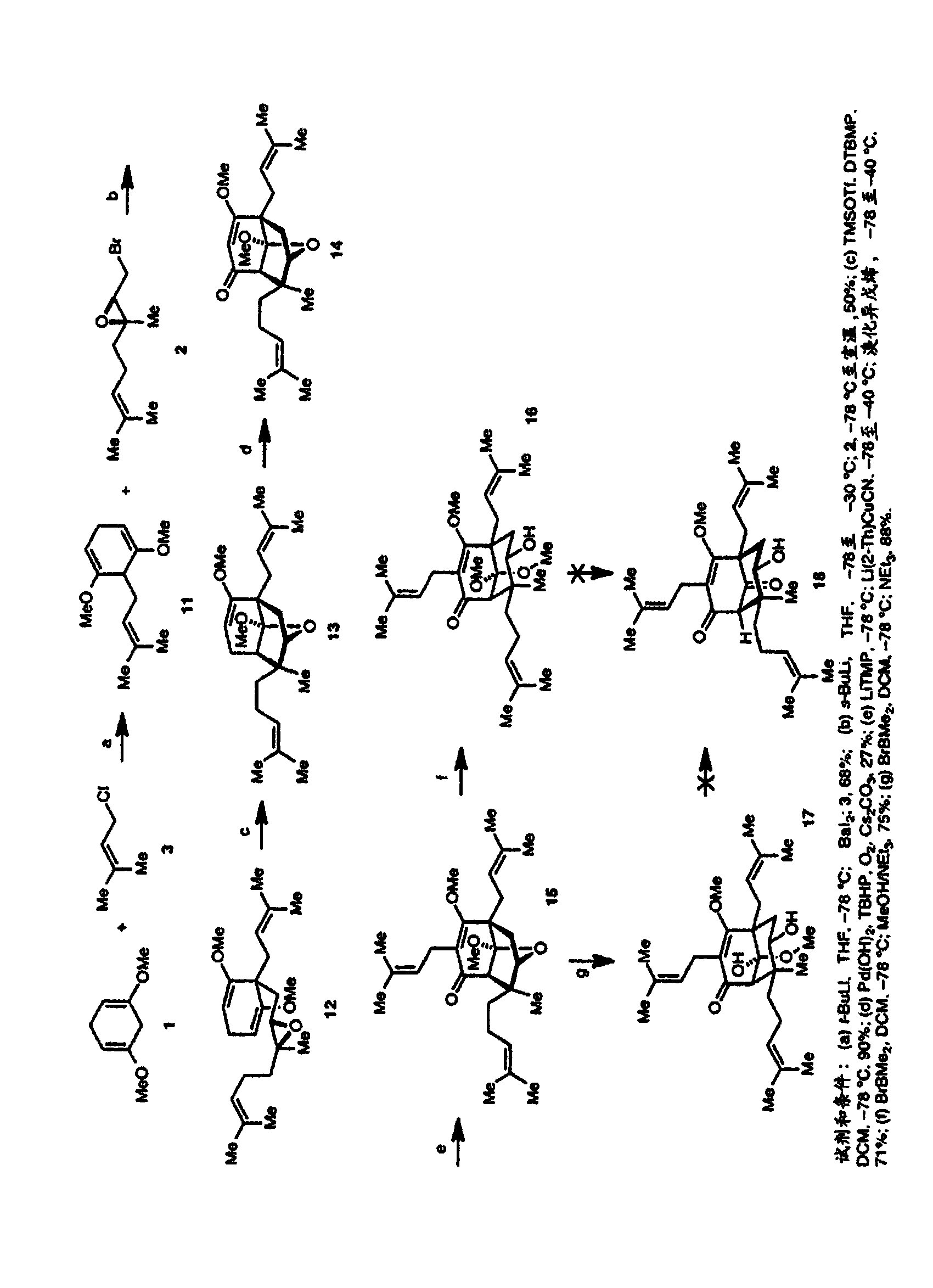
[0495] The criteria for the development of an enantioselective synthesis of hyperforin were that it must be very simple (about 10-15 steps), involve minimal changes in oxidation state, be modular, and be derived from starting materials that are readily available . By satisfying these criteria, we can easily obtain many hyperforin analogs for biological research. The retrosynthesis of hypericin is shown in figure 1 . The first simplification is the derivation of hyperforin from an intermediate in which the key C2 and C5 quaternary atomic centers are set. We envisage that this intermediate will be derived from the Lewis acid-catalyzed 6-endo-tet cyclization of enol ethers to trisubstituted epoxides. W...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com