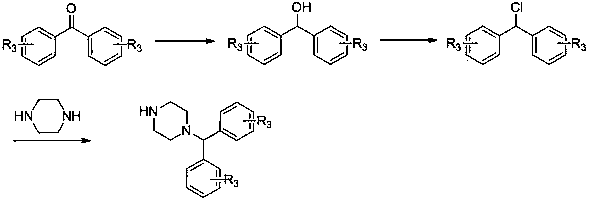
Aryl substituted piperazine carbonyl derivative as well as preparation method and application thereof
A derivative, aryl technology, applied in the pharmaceutical field, can solve the problems of poor oral bioavailability, low neurobehavioral toxicity, induced bleeding, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1 Ethyl 2-methylphenoxyacetate
[0055] Add 2-methylphenol (5.3g, 0.0490mol), ethyl chloroacetate (6.3mL, 0.0588mol), acetonitrile (30mL) and potassium carbonate (6.8g, 0.0490mol) into a 100mL eggplant-shaped flask, heat up to reflux , After reacting for 5 hours, it was filtered while it was hot, and the filtrate was spin-dried. The raffinate was distilled under reduced pressure, and the fraction at 102-106°C / 0.08mmHg was collected to obtain 6.0 g of a clear oil with a yield of 63.0%, and TLC showed a pure point.
Embodiment 2
[0056] Example 2 Ethyl 4-methylphenoxyacetate
[0057] Add 4-methylphenol (5.3g, 0.0490mol), ethyl chloroacetate (6.3mL, 0.0588mol), acetonitrile (30mL) and potassium carbonate (6.8g, 0.0490mol) into a 100mL eggplant-shaped flask, heat up to reflux , After reacting for 5 hours, it was filtered while it was hot, and the filtrate was spin-dried. The raffinate was distilled under reduced pressure, and the fraction at 110-114°C / 0.08mmHg was collected to obtain 5.6g of a clear oil with a yield of 58.0%, and TLC showed a pure point.
Embodiment 3
[0058] Example 3 2-(2-naphthyloxy) ethyl acetate
[0059] Add ethyl naphthol (7.1g, 0.0490mol), ethyl chloroacetate (6.3mL, 0.0588mol), acetonitrile (30mL) and potassium carbonate (6.8g, 0.0490mol) into a 100mL eggplant-shaped bottle, raise the temperature to reflux, and react After 5 hours, it was filtered while it was hot, the filtrate was spin-dried, left to cool, and the oil was separated into a solid, which was recrystallized with absolute ethanol to obtain 10 g of a light pink solid with a yield of 88.5%, and TLC showed a pure point.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com