Method for synthesizing 9-aryl fluorene compound
A technology for aromatic hydrocarbon compounds and compounds, which is applied in the field of synthesizing 9-arylfluorene compounds, can solve the problems of high price, high cost, and harsh reaction temperature conditions of 130°C, and achieve simple operation, small environmental impact, and less harsh reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Synthesis of 9-phenylfluorene:
[0031] Add 91mg of 2-phenylbenzaldehyde (0.5mmol), 5ml of benzene and 4.4μL of trifluoromethanesulfonic acid (0.05mmol) to the test tube successively, stir for one minute, add 95μL of acetic anhydride (1mmol), and react at room temperature for 24h to obtain Reaction mixture (reaction time is determined according to thin-layer chromatographic analysis, stop when the reaction of raw materials is complete), the obtained reaction mixture is used to remove the solvent by vacuum distillation, and column chromatography (eluent is pure petroleum ether) is used to react crude oil. The product is purified to obtain the product. The yield was 28%.
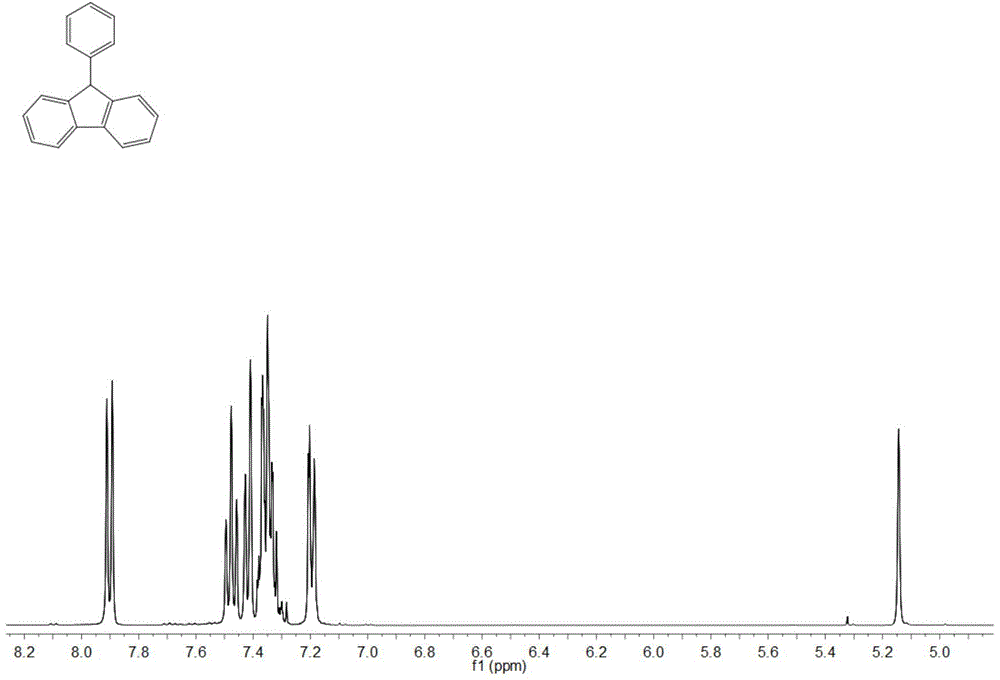
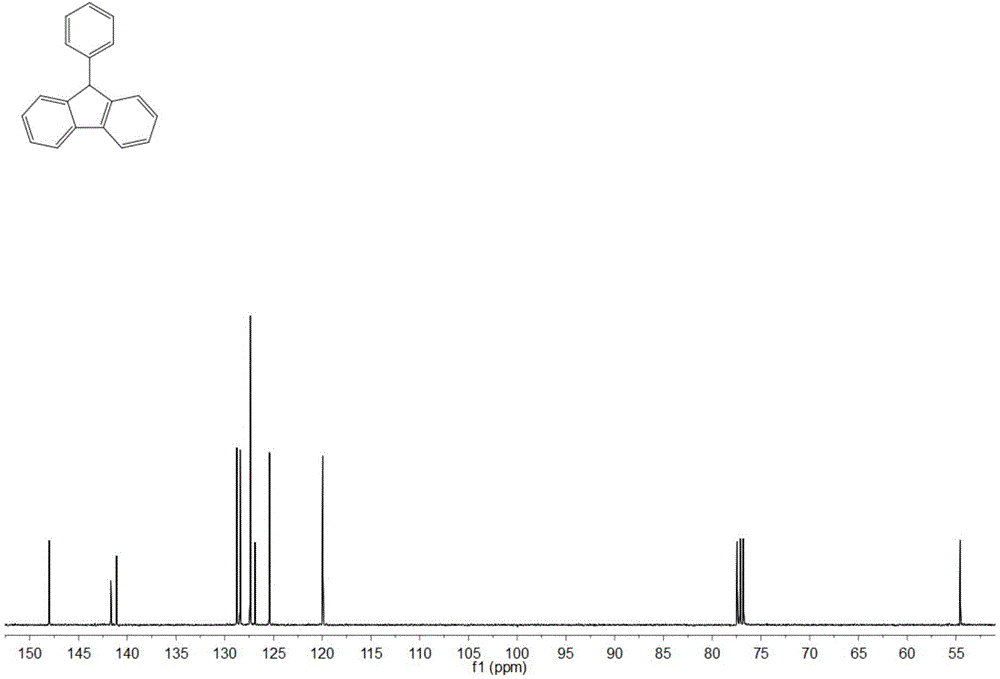
[0032] Adopt nuclear magnetic resonance (NMR) method to test the product prepared in this embodiment, such as figure 1 and figure 2 , are respectively the product obtained in this example 1 H NMR spectrum and 13 C NMR spectrum, where 1 H NMR (400MHz, CDCl 3 ) data are: δ7.90(2H,d,J7.6),7.48(2H,t...
Embodiment 2
[0037] Synthesis of 2-tert-butyl-9-(2,4,6-trimethylphenyl)fluorene:
[0038] Add 5 mL of 1,2-dichloroethane, 119 mg of 2-(4-tert-butylphenyl)benzaldehyde (0.5 mmol), 348 μL of 1,3,5-trimethylbenzene (2.5 mmol) and 4.4 μL of trifluoroform to the test tube in sequence Sulfuric acid (0.05mmol), after stirring for one minute, add 95μL acetic anhydride (1mmol), and react at room temperature for 17h (the reaction time is determined by thin-layer chromatography analysis, stop when the reaction of raw materials is complete). The resulting reaction mixture was distilled under reduced pressure to remove the solvent, and the crude reaction product was purified by column chromatography (the eluent was pure petroleum ether) to obtain the product. The product yield is greater than 99%.
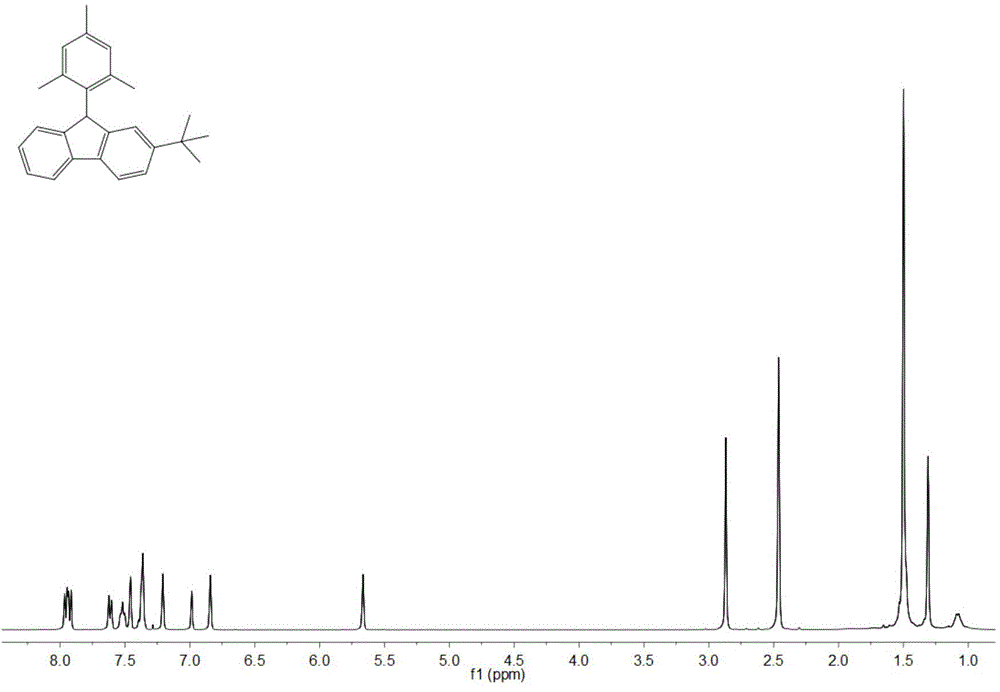
[0039] Adopt nuclear magnetic resonance (NMR) method to test the product prepared in this embodiment, such as image 3 and Figure 4 , are respectively the product obtained in this example 1 H NMR spect...
Embodiment 3
[0042] Synthesis of 9-(9-anthracenyl)fluorene:
[0043] Add 5mL of 1,2-dichloroethane, 91mg (0.5mmol) of 2-phenylbenzaldehyde, 96mg (0.54mmol) of anthracene and 4.4μL of trifluoromethanesulfonic acid (0.05mmol) to the test tube, and stir for one minute Add 95 μL of acetic anhydride (1 mmol), react at room temperature for 4 hours (the reaction time is determined by thin-layer chromatography analysis, stop when the reaction of the raw materials is complete), the obtained reaction mixture is removed by vacuum distillation, and column chromatography (elution The liquid is pure petroleum ether) to purify the reaction crude product to obtain the product. The product yield was 88%.
[0044] Adopt nuclear magnetic resonance (NMR) method to test the product prepared in this embodiment, such as Figure 5 and Image 6 , are respectively the product obtained in this example 1 H NMR spectrum and 13 C NMR spectrum, where 1 H NMR (400MHz, CDCl 3 ) data are: δ8.48(1H,d,J8.9),8.28(1H,s)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com