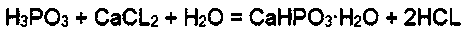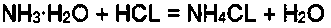Preparation method of calcium phosphite
A technology of calcium phosphite and phosphorous acid, which is applied in the direction of phosphorous acid, phosphorus oxyacid, ammonium halide, etc., can solve the problems of not being able to meet industrial production, high requirements for reaction conditions, and low product purity, and meet the requirements of reaction conditions Low, high product purity, no effect of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The preparation method of calcium phosphite, the reaction steps are: first, the mass concentration of 50% phosphorous acid and the mass concentration of 50% calcium chloride are added into the reactor in a molar ratio of 1:1 for reaction, and the reaction temperature Control it within the range of 50-80°C. After the phosphorous acid and calcium chloride have reacted by 30%, start to drop ammonia water with a mass concentration of 20% into the reactor according to the molar ratio of phosphorous acid to 2:1. The ammonia water is added dropwise completely, and the reaction ends; then, the solid-liquid separation is carried out on the reactant, and the separated solid phosphorous acid is washed with water and dried to obtain the finished product. The separated liquid is ammonium chloride aqueous solution, which can be used to prepare fertilizers, and no three wastes are generated during the whole reaction process, and the reaction process is safe and environment-friendly.
Embodiment 2
[0019] The preparation method of calcium phosphite, the reaction steps are: firstly add phosphorous acid with a mass concentration of 40% and calcium chloride with a mass concentration of 50% in a molar ratio of 1:1 into the reactor for reaction, the reaction temperature Control it within the range of 50-80°C. After the phosphorous acid and calcium chloride have reacted by 20%, start adding ammonia water with a mass concentration of 30% into the reactor at a molar ratio of 2.1:1 to the phosphorous acid. The ammonia water is added dropwise completely, and the reaction ends; then, the solid-liquid separation is carried out on the reactant, and the separated solid phosphorous acid is washed with water and dried to obtain the finished product. The separated liquid is ammonium chloride aqueous solution, which can be used to prepare fertilizers, and no three wastes are generated during the whole reaction process, and the reaction process is safe and environment-friendly.
Embodiment 3
[0021] The preparation method of calcium phosphite, the reaction steps are as follows: first, the mass concentration of 40% phosphorous acid and the mass concentration of 40% calcium chloride are added into the reactor in a molar ratio of 1:1 for reaction, and the reaction temperature Control it within the range of 50-80°C. After the phosphorous acid and calcium chloride have reacted by 40%, start adding ammonia water with a mass concentration of 10% into the reactor at a molar ratio of 2:1 to the phosphorous acid. The ammonia water is added dropwise completely, and the reaction ends; then, the solid-liquid separation is carried out on the reactant, and the separated solid phosphorous acid is washed with water and dried to obtain the finished product. The separated liquid is ammonium chloride aqueous solution, which can be used to prepare fertilizers, and no three wastes are generated during the whole reaction process, and the reaction process is safe and environment-friendly. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com