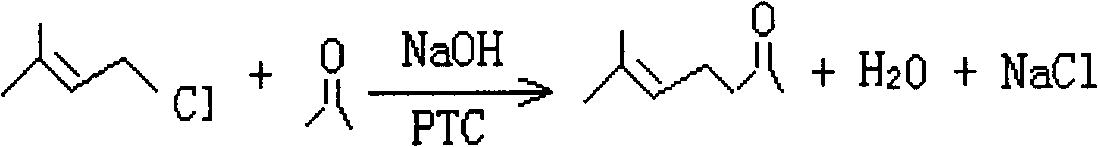Preparation method of methyl heptenone
A technology of methyl heptenone and acetone, which is applied in the field of preparation of methyl heptenone, can solve the problem that the catalyst cannot be recycled, achieve high yield, simplify the process, and reduce the effect of process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~10
[0020] Add benzyltriethylammonium chloride catalyst, chlorinated isopentene and acetone in the tank reactor, under alkaline conditions, chlorinated isopentene and acetone undergo condensation reaction, after the reaction is completed, the lye is evaporated and concentrated apply. The hydrogenation reaction conditions of each embodiment are shown in Table 1. The content of methyl heptenone in the condensation reaction liquid was determined by gas chromatography, and the conversion rate and selectivity of chloroisoamylene and the yield of methyl heptenone were calculated. The results are shown in Table 2.
[0021] Table 1.
[0022]
[0023]
[0024] Table 2.
[0025]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com