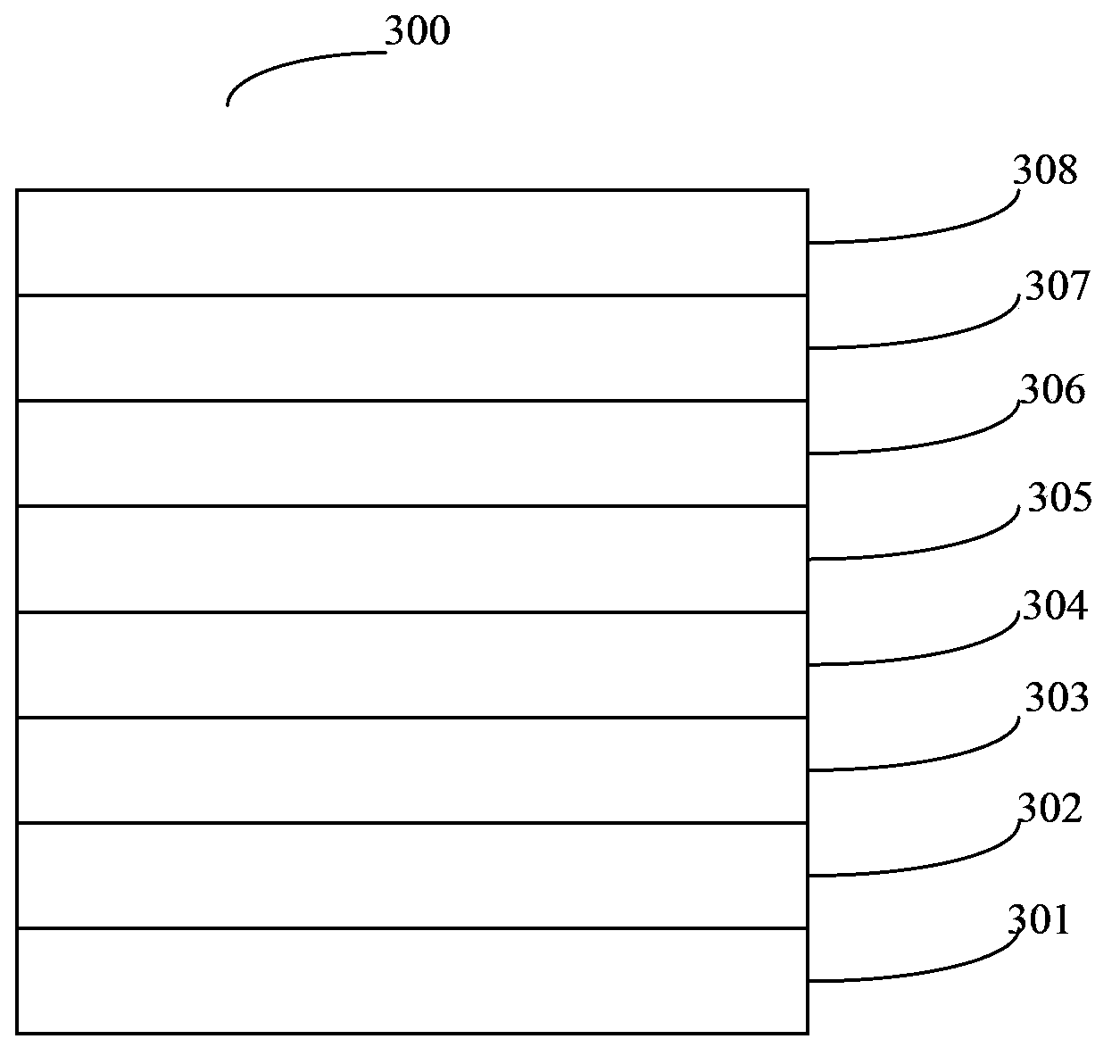
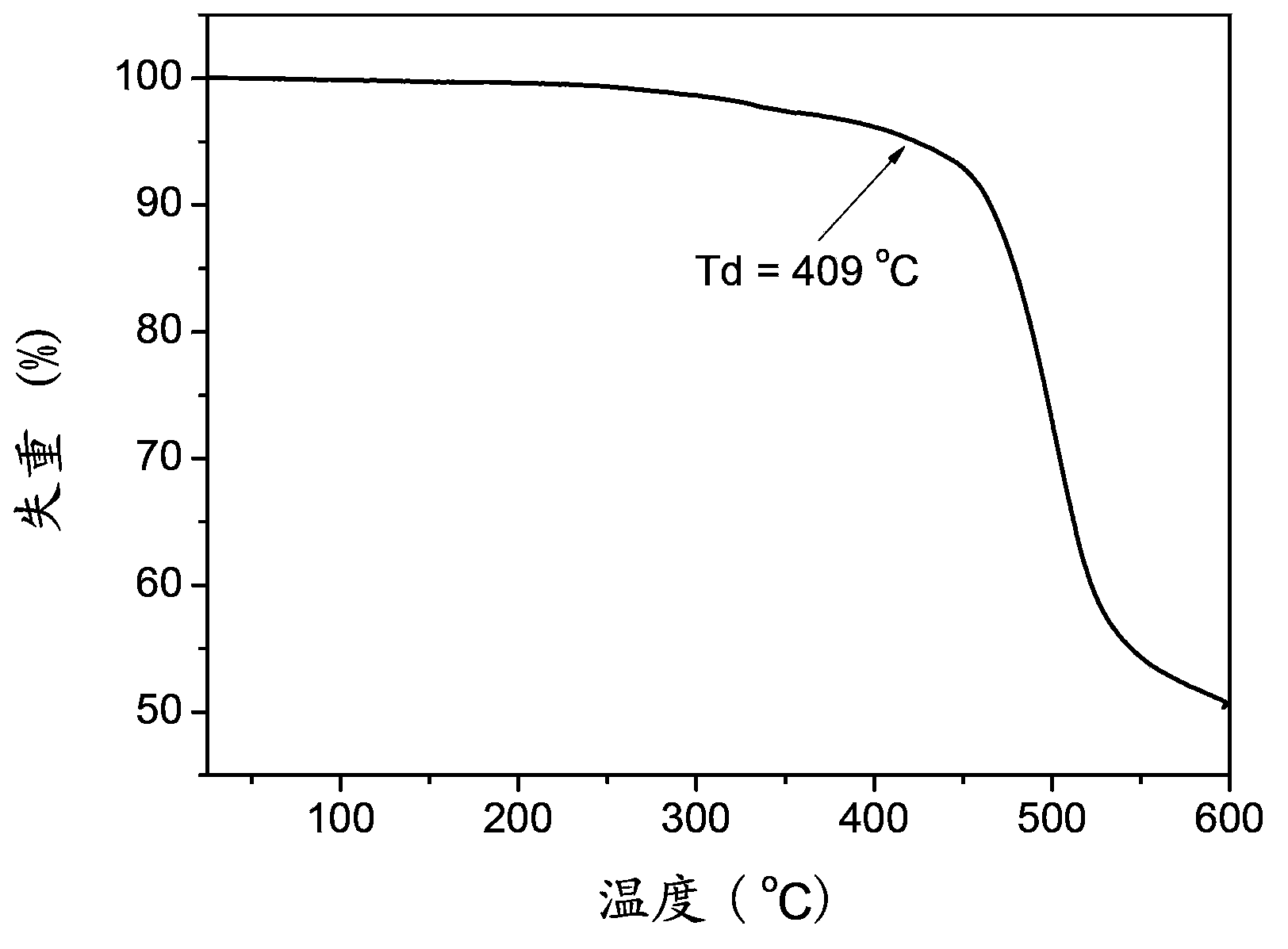
Organic semiconductor material, preparation method of organic semiconductor material and electroluminescent device
A technology of electroluminescent devices and organic semiconductors, which is applied in the direction of semiconductor devices, semiconductor/solid-state device manufacturing, and preparation of organic compounds, etc., can solve problems such as lack, achieve high triplet energy levels, improve luminous efficiency, and simple synthesis methods easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
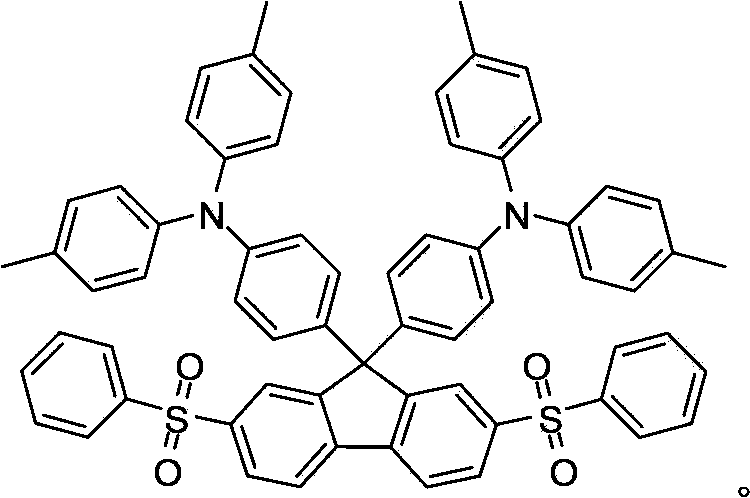
[0023] Preparation process of 2,7-bis(phenylsulfoxy)-9,9-bis(4-(di-p-tolyl)aminophenyl)fluorene 1. Compound D: 2,7-dibromo-9,9 - Bis(4-(di-p-tolyl)aminophenyl)fluorene structural formula is The synthesis is as follows:
[0024]
[0025] Compound A: 2,7-dibromofluorenone (5.4g, 16mmol) was mixed with compound B: 4,4'-dimethyltriphenylamine (15g 55mmol), heated to 120°C under nitrogen atmosphere, and the mixture was completely dissolved , 1 mL of catalyst trifluoromethanesulfonic acid was added dropwise, and heating was maintained overnight. After the reaction, dichloromethane was added to dissolve the unreacted raw materials and other impurities, and the white solid compound D was obtained by filtration: 2,7-dibromo-9,9-bis(4-(di-p-tolyl)aminophenyl)fluorene , and the yield was 86%.
[0026] Experimental test data: mass spectrum: m / z867.2 (M + +1); elemental analysis (%) C 53 h 42 Br 2 N 2 : Theoretical value C73.45, H4.88, N3.23; measured value: C73.34, H4.90, N3.2...
Embodiment 2
[0032] Example 2: Preparation process of 2,7-bis(phenylsulfoxy)-9,9-bis(4-(di-p-tolyl)aminophenyl)fluorene
[0033] 1. Compound D: Refer to Example 1 for the preparation of 2,7-dibromo-9,9-bis(4-(di-p-tolyl)aminophenyl)fluorene.
[0034] 2. The structural formula of 2,7-bis(phenylsulfoxy)-9,9-bis(4-(di-p-tolyl)aminophenyl)fluorene is The synthesis is as follows:
[0035]
[0036] Compound D: 2,7-dibromo-9,9-bis(4-(di-p-tolyl)aminophenyl)fluorene (7.8 g, 9 mmol) was dissolved in potassium carbonate (2.12 g, 20.0 mmol) in toluene (150mL) solution, then add compound C: thiophenol (2.3g, 20.7mmol) and mix in a three-necked flask, stir and react at 110°C for 10 hours, stop the reaction and cool to room temperature, extract with dichloromethane, and rotate to evaporate The solvent was removed to obtain a crude product, which was then dissolved in acetic acid (100 mL), and 30% by mass hydrogen peroxide (50 mL) was added, and the reaction was stirred at 80°C for 24 hours. Wash ...
Embodiment 3
[0037] Example 3: Preparation process of 2,7-bis(phenylsulfoxy)-9,9-bis(4-(di-p-tolyl)aminophenyl)fluorene
[0038] 1. Compound D: Refer to Example 1 for the preparation of 2,7-dibromo-9,9-bis(4-(di-p-tolyl)aminophenyl)fluorene.
[0039]2. The structural formula of 2,7-bis(phenylsulfoxy)-9,9-bis(4-(di-p-tolyl)aminophenyl)fluorene is The synthesis is as follows:
[0040]
[0041] Compound D: 2,7-dibromo-9,9-bis(4-(di-p-tolyl)aminophenyl)fluorene (7.8 g, 9 mmol) was dissolved in tetrahydrofuran of potassium carbonate (1.68 g, 20.0 mmol) (150mL) solution, then add compound C: thiophenol (2.4g, 21.6mmol) and mix in a three-necked flask, stir and react at 70°C for 12 hours, stop the reaction and cool to room temperature, extract with dichloromethane, and rotate to evaporate The solvent was removed to obtain a crude product, which was then dissolved in acetic acid (100 mL), and 30% by mass hydrogen peroxide (50 mL) was added, and the reaction was stirred at 80°C for 24 hours. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| electrical resistance | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com