Pharmaceutical composition capable of treating alzheimer's disease
A technology for Alzheimer's disease and composition, which is applied in the field of pharmaceutical compositions capable of treating Alzheimer's disease, and can solve problems such as undiscovered
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
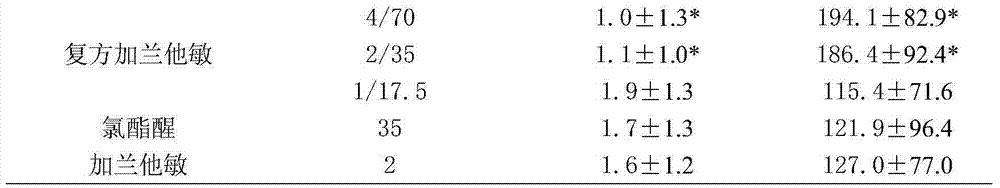
[0011] The design of this embodiment is a pharmaceutical composition capable of treating Alzheimer's disease, which is composed of galantamine and chlorpheniramine. The compatibility ratio of galantamine and perchlorate is (0.5-4): (17.5-70).
[0012] 1.1 Mouse platform method to determine the effect and compatibility ratio of compound galantamine on Alzheimer's disease
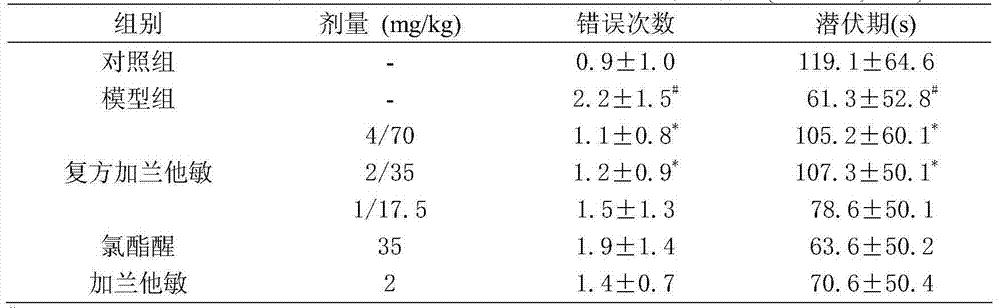
[0013] Take healthy male Kunming mice, and then divide them into 21 groups, including 12 compound groups, 7 single groups, normal control group and model group, normal control group and model group, and the normal control group and model group are given equal volumes of distilled water. Feed galantamine 4, 2, 1, 0.5mg / kg, chlorpheniramine 70, 35, 17.5mg / kg, galantamine / chlorpheniramine 4 / 70, 4 / 35, 4 / 17.5, 2 / 70, 2 / 35, 2 / 17.5, 1 / 70, 1 / 35, 1 / 17.5, 0.5 / 70, 0.5 / 35, 0.5 / 17.5mg / kg, continuous administration for 7 days. 20 minutes after the last administration, except for the normal control group, which was intrap...
Embodiment 2
[0019] Screening of the optimal ratio of galantamine and chlorpheniramine.
[0020] 2.1 Mouse platform method to determine the best ratio
[0021] Experimental animals and methods are the same as 1.1.
[0022] Orthogonal experimental design and Q 50 Methods Dosage design was carried out and analysis of variance was used to analyze the experimental results. There was a synergistic effect between galantamine and chlorpheniramine (F50 method), the Q value greater than 1 means that the combined effect of the formula is greater than the sum of the single effects. The results showed that the optimal dose ratio of galantamine / chlorpheniramine was 2 / 35mg / kg, and the Q value was the largest Q=1.77.
[0023] 2.2 Determining the best ratio by mouse avoidance method
[0024] Experimental animals and methods are the same as 1.2.
[0025] Orthogonal experimental design and Q 50 Methods The dosage was designed and the experimental results were analyzed by analysis of variance. There was...
Embodiment 3
[0028] This example relates to the improving effect of compound galantamine on memory acquisition disorder, memory consolidation disorder and memory reproduction disorder. .
[0029] 3.1 Improvement effect of compound galantamine on scopolamine-induced memory impairment in mice (stepping method)
[0030] Take healthy male Kunming mice, and then divide them into 7 groups, namely normal control group, model group, compound galantamine (COG) 4 / 70, 2 / 35, 1 / 17.5 mg / kg group, and chlorpheniramine 35 mg / kg group and galantamine 2mg / kg group. Except the normal control group and the model group were given equal volumes of distilled water, the rest of the groups were given corresponding drugs for 7 consecutive days. 20 minutes after the last administration, except for the normal control group, which was intraperitoneally injected with normal saline, the rest of the groups were intraperitoneally injected with 5 mg / kg of scopolamine, and 10 minutes later, they performed platform traini...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com