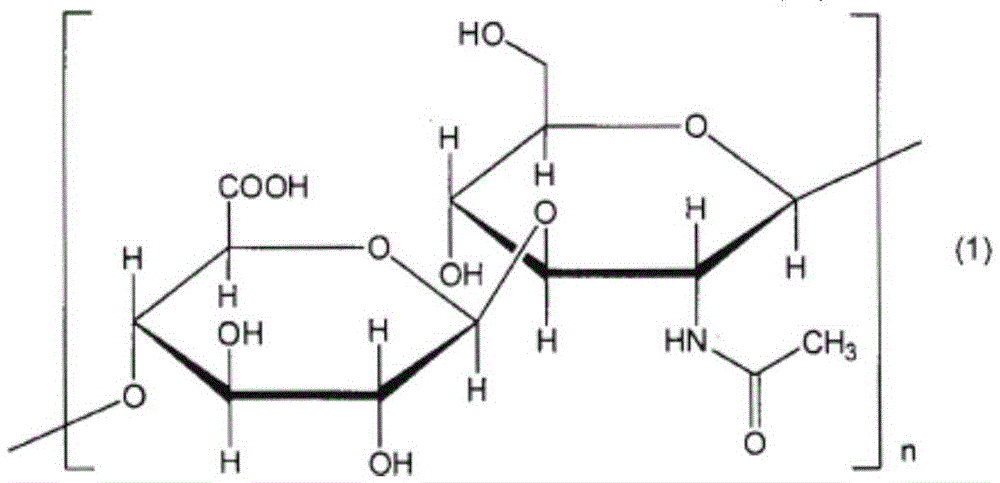
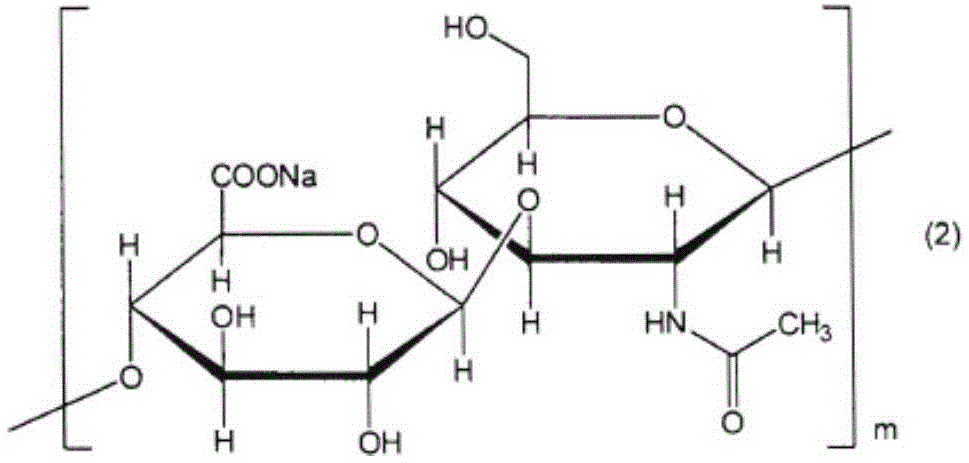
Eye drops containing hyaluronic acid or its salts and propylene glycol
A technology of hyaluronic acid and sodium hyaluronate, which is applied to medical preparations containing active ingredients, medical preparations with non-active ingredients, organic active ingredients, etc. It can solve the problems of no record and insufficient preservation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
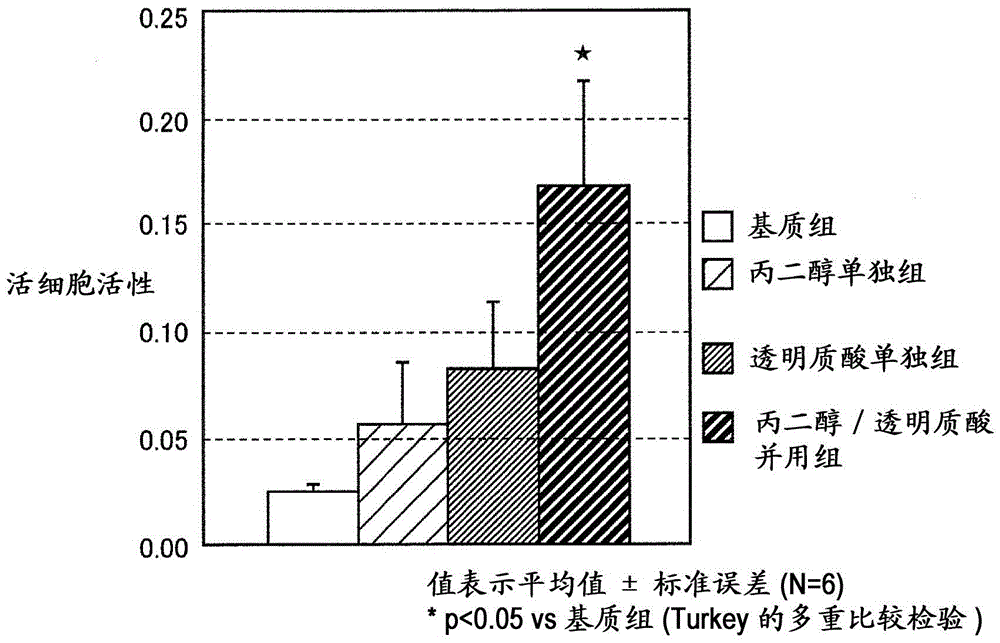
[0072] [preservation effectiveness test]
[0073] In order to confirm the effect of propylene glycol on the preservation efficacy of hyaluronic acid eye drops, a preservation efficacy test was conducted.
[0074] (sample preparation)
[0075]
[0076] Dissolve sodium hyaluronate 0.3g, sodium chloride 0.7g, potassium chloride 0.15g, ε-aminocaproic acid 0.2g, edetate sodium hydrate 0.01g and benzalkonium chloride (C12) 0.0025g in water, Set the volume to 100mL, add dilute hydrochloric acid and / or sodium hydroxide, use the liquid with pH 6.0 as comparative formula 1, and use the liquid with pH 7.0 as comparative formula 2.
[0077]
[0078] Except the addition amount of benzalkonium chloride (C12) being 0.002 g, it prepared similarly to said comparative formulation 1 and 2.
[0079]
[0080] Dissolve sodium hyaluronate 0.3g, sodium chloride 0.7g, propylene glycol 0.25g, ε-aminocaproic acid 0.2g, edetate sodium hydrate 0.01g and benzalkonium chloride (C12) 0.001g in water,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com