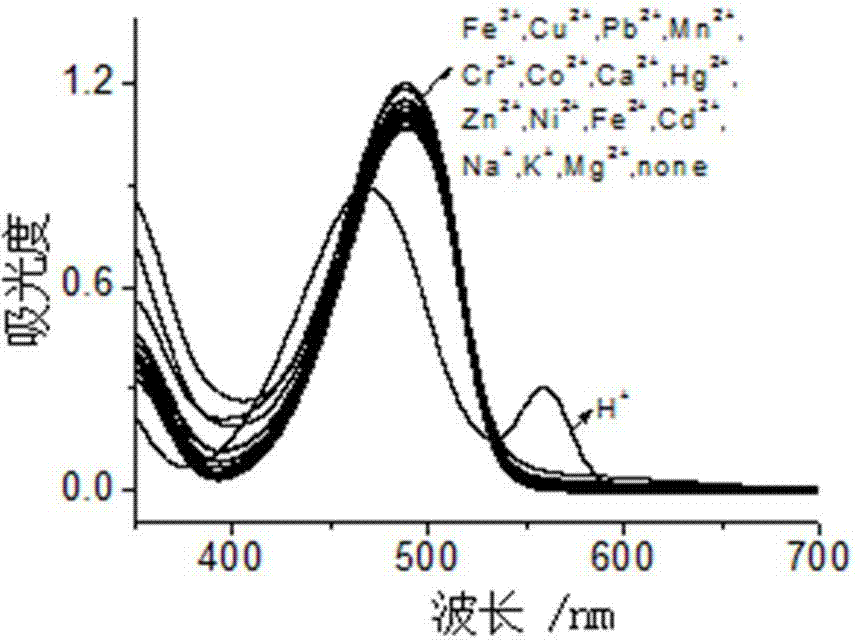
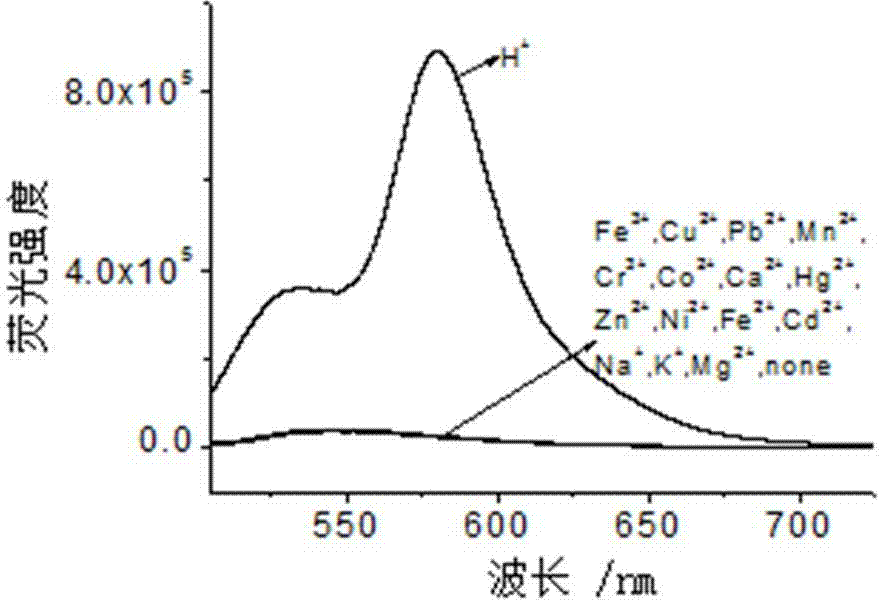
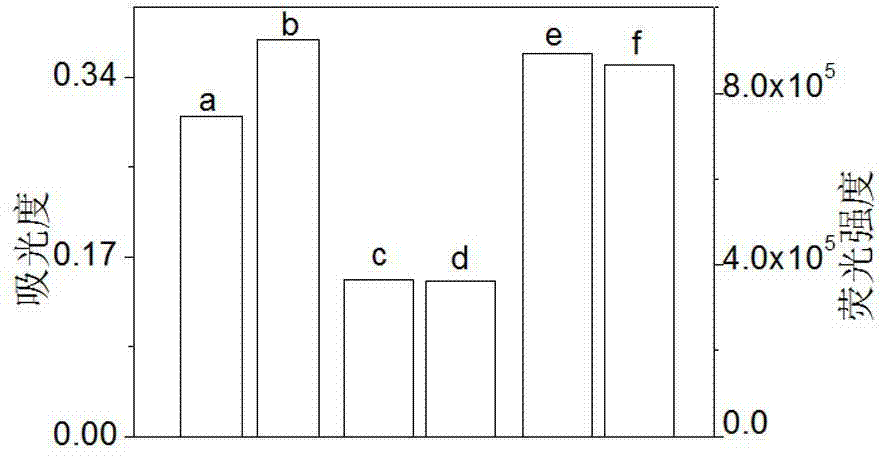
Rhodamine-oxadiazole derivative and preparation method and application thereof
A technology of oxadiazole and derivatives, which is applied in the field of organic synthesis, can solve the problems of low total yield of rhodamine derivatives and cumbersome reaction process, and achieve the effects of avoiding fluorescence resonance energy transfer, high reaction efficiency and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment one: H + Preparation of probe rhodamine-oxadiazole derivatives (RPBD)
[0033] N-aminoethylpiperazine (AEP), rhodamine B (RB), DIPEA, 4-chloro-7-nitro-2,1,3-benzoxadiazole (NBD-Cl), K 2 CO 3 The molar ratio is: 1:1:1:1:1. in N 2 Under protection, AEP and DIPEA were dissolved in acetonitrile in a 100 mL three-neck flask, RB was dissolved in acetonitrile and added dropwise to the above solution, stirred at room temperature for 30 min, then heated to reflux for 12 h, then cooled, and rotary evaporated Acetonitrile was removed, the residue was washed with water and dissolved in acetonitrile, and K 2 CO 3 , then NBD-Cl dissolved in the same solvent was added dropwise to the above solution, reacted at room temperature for 30 min, the solvent was removed by rotary evaporation, and the residue was separated by silica gel column chromatography, developing solvent: ethyl acetate / petroleum ether (vol. Ratio 2 / 1), the orange-red solid target product RPBD was obtain...
Embodiment 2
[0035] Embodiment two: H + Preparation of probe RPBD
[0036] N-aminoethylpiperazine (AEP), rhodamine B (RB), DIPEA, 4-chloro-7-nitro-2,1,3-benzoxadiazole (NBD-Cl), K 2 CO 3 The molar ratio is: 7:1:12:1:1. in N 2 Under protection, AEP and DIPEA were dissolved in acetonitrile in a 100 mL three-neck flask, RB was dissolved in acetonitrile and added dropwise to the above solution, stirred at room temperature for 30 min, then heated to reflux for 12 h, then cooled, and rotary evaporated Acetonitrile was removed, the residue was washed with water and dissolved in acetonitrile, and K 2 CO 3 , then NBD-Cl dissolved in the same solvent was added dropwise to the above solution, reacted at room temperature for 30 min, the solvent was removed by rotary evaporation, and the residue was separated by silica gel column chromatography, developer: ethyl acetate / petroleum ether (vol. Ratio 2 / 1), the orange-red solid target product RPBD was obtained with a yield of 82.5%.
Embodiment 3
[0037] Embodiment three: H + Preparation of probe RPBD
[0038] N-aminoethylpiperazine (AEP), rhodamine B (RB), DIPEA, 4-chloro-7-nitro-2,1,3-benzoxadiazole (NBD-Cl), K 2 CO 3 The molar ratio is: 10:1:20:1:1. in N 2 Under protection, AEP and DIPEA were dissolved in acetonitrile in a 100 mL three-neck flask, RB was dissolved in acetonitrile and added dropwise to the above solution, stirred at room temperature for 30 min, then heated to reflux for 12 h, then cooled, and rotary evaporated Acetonitrile was removed, the residue was washed with water and dissolved in acetonitrile, and K 2 CO 3 , then NBD-Cl dissolved in the same solvent was added dropwise to the above solution, reacted at room temperature for 30 min, the solvent was removed by rotary evaporation, and the residue was separated by silica gel column chromatography, developer: ethyl acetate / petroleum ether (vol. Ratio 2 / 1), the orange-red solid target product RPBD was obtained with a yield of 78.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com