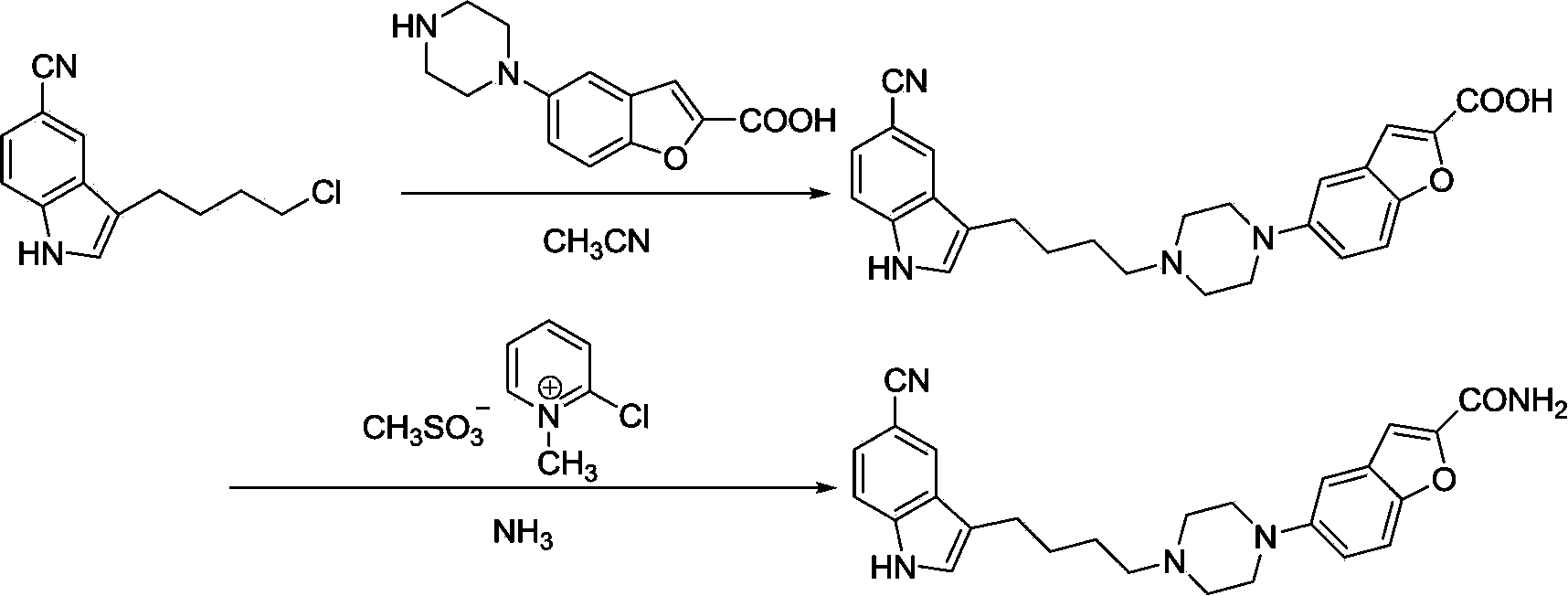
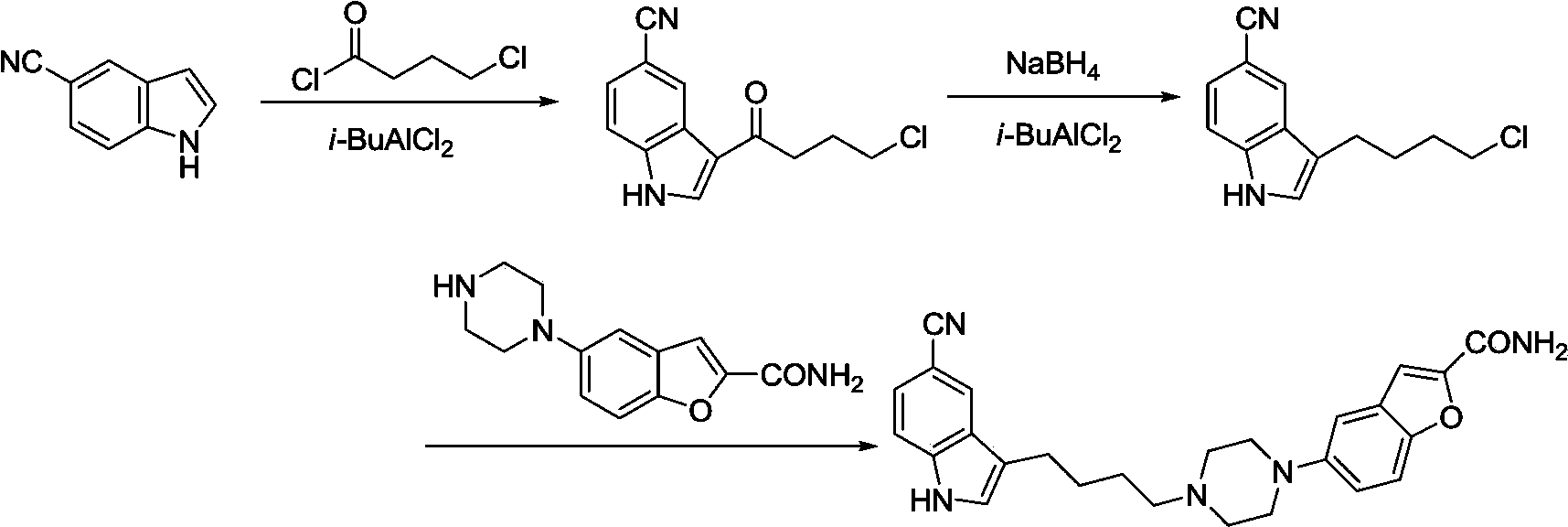
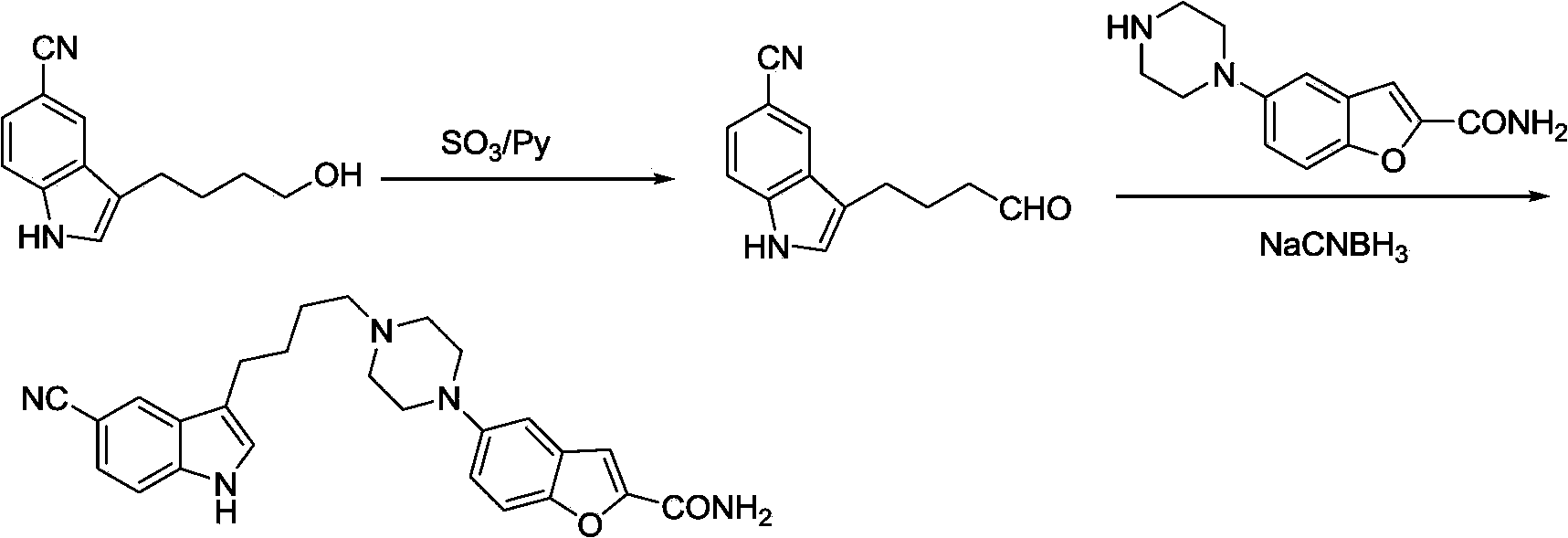
Method for preparing vilazodone and intermediate thereof
A compound, selected technology, applied in the direction of organic chemistry, bulk chemical production, etc., can solve the problems of harsh Buchwald reaction conditions, not suitable for mass industrial production, and low reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099]
[0100] (1) Preparation of 5-(4-(3-butenyl)piperazin-1-yl)benzofuran-2-carboxamide
[0101] Add 24.5g (100mmol) of 5-(piperazin-1-yl)benzofuran-2-carboxamide, 41.4g (300mmol) of potassium carbonate, and 400ml of N,N-dimethylformamide in the reaction flask, at room temperature 16.2g (120mmol) of 4-bromo-1-butene was added dropwise, and then heated to 60°C and stirred for 12h. Stop heating, cool to room temperature, filter, add the filtrate to 800 ml of water, stir for 0.5 h, filter, dry, and recrystallize with ethyl acetate to obtain 25.6 g of a brown solid with a yield of 86%.
[0102] MS-ESI(M+1): 300
[0103] 1 H NMR: (400MHz, d-DMSO) δ: 8.05 (s, 1H), 7.63 (s, 1H), 7.47 (d, J=9.6 Hz, 1H), 7.41 (s, 1H), 5.83 (m, 1H) ), 5.08(dd, J=17.2, 1.6Hz, 1H), 5.00(d, J=8.0Hz, 1H), 3.11(t, J=4.8Hz, 4H), 2.55(t, J=4.8Hz, 4H ), 2.41(t, J=7.2Hz, 2H), 2.35(m, 2H)
[0104] (2) 5-(4-(4-(1-benzyl-5-cyanoindol-3-yl)-3-but-enyl)piperazin-1-yl)benzofuran-2-methyl Preparation of amide
[0105] Ad...
Embodiment 2
[0113]
[0114] (1) Preparation of 5-(4-(3-butenyl)piperazin-1-yl)benzofuran-2-carboxamide
[0115] Add 24.5g (100mmol) of 5-(piperazin-1-yl)benzofuran-2-carboxamide, 42.4g (200mmol) of potassium phosphate, and 400ml of tetrahydrofuran into the reaction flask, and add 4-iodine-1 dropwise at room temperature. -Butene 18.2g (120mmol), then heated to 70°C and stirred for 36h. Stop heating, cool to room temperature, filter, add the filtrate to 1000 ml of water, stir for 1 h, filter, dry, and recrystallize with ethyl acetate to obtain 22.8 g of brown solid with a yield of 76.3%.
[0116] (2) 3-(4-(4-(2-carbamoylbenzofuran-5-yl)piperazin-1-yl)-1-butenyl)-5-cyanoindole-1-carboxylic acid Preparation of tert-butyl ester
[0117] Add 22.8g (76.8mmol) of 5-(4-(3-butenyl)piperazin-1-yl)benzofuran-2-carboxamide, 5-cyano-3-iodoindole- 23.4g (63.5mmol) of tert-butyl 1-formate, 0.58g (0.5mmol) of tetrakis(triphenylphosphine)palladium and 150ml of N,N-dimethylformamide, replaced with argon for thr...
Embodiment 3
[0126]
[0127] (1) Preparation of 5-(4-(3-butenyl)piperazin-1-yl)benzofuran-2-carboxamide
[0128] Add 24.5g (100mmol) of 5-(piperazin-1-yl)benzofuran-2-carboxamide, 20.2g (200mmol) of triethylamine, and 400ml of N,N-dimethylformamide into the reaction flask, 27.3g (150mmol) of 4-bromo-1-butene was added dropwise at room temperature, and then heated to 60°C and stirred for 6h. Stop heating, cool to room temperature, filter, add the filtrate to 800 ml of water, stir for 0.5 h, filter, dry, and recrystallize with ethyl acetate to obtain 24.1 g of a brown solid with a yield of 80.6%.
[0129] (2) Preparation of 5-(4-(4-(5-cyanoindol-3-yl)-3-butenyl)-piperazin-1-yl)benzofuran-2-carboxamide
[0130] Add 24.0g (80.1mmol) of 5-(4-(3-butenyl)piperazin-1-yl)benzofuran-2-carboxamide and 18.0 of 3-bromoindole-5-carbonitrile to the reaction flask. g (81.4mmol), triethylamine 24.3g (240.3mmol), bis(triphenylphosphine) palladium dichloride 0.701g (1mmol) and tris(o-methylphenyl)phosphine 0.6g, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com