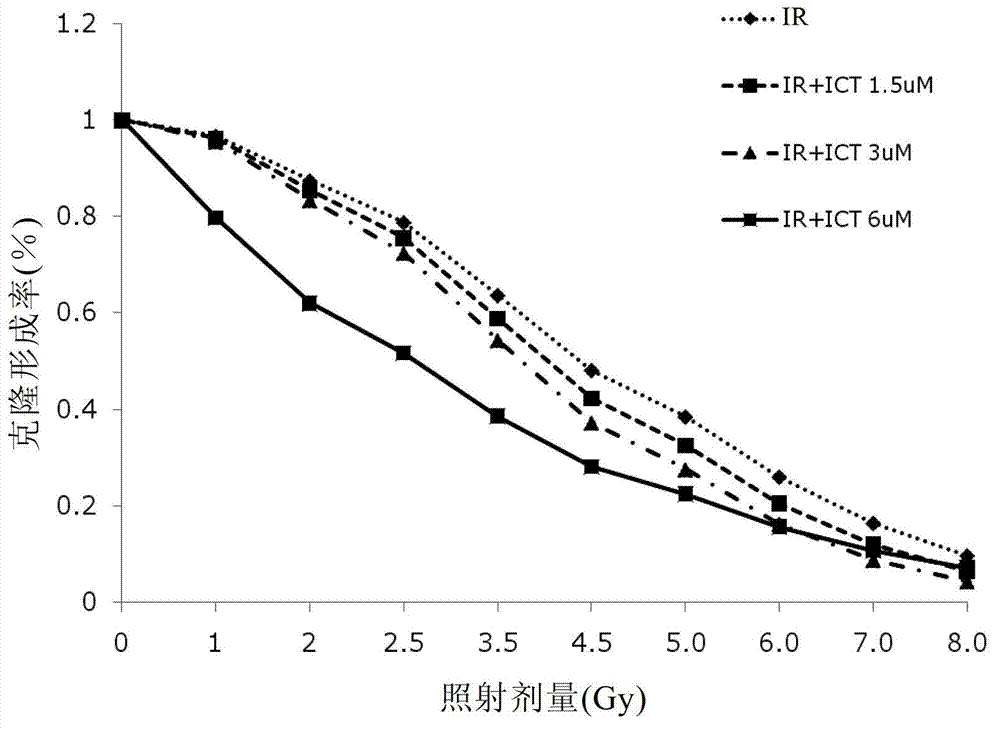
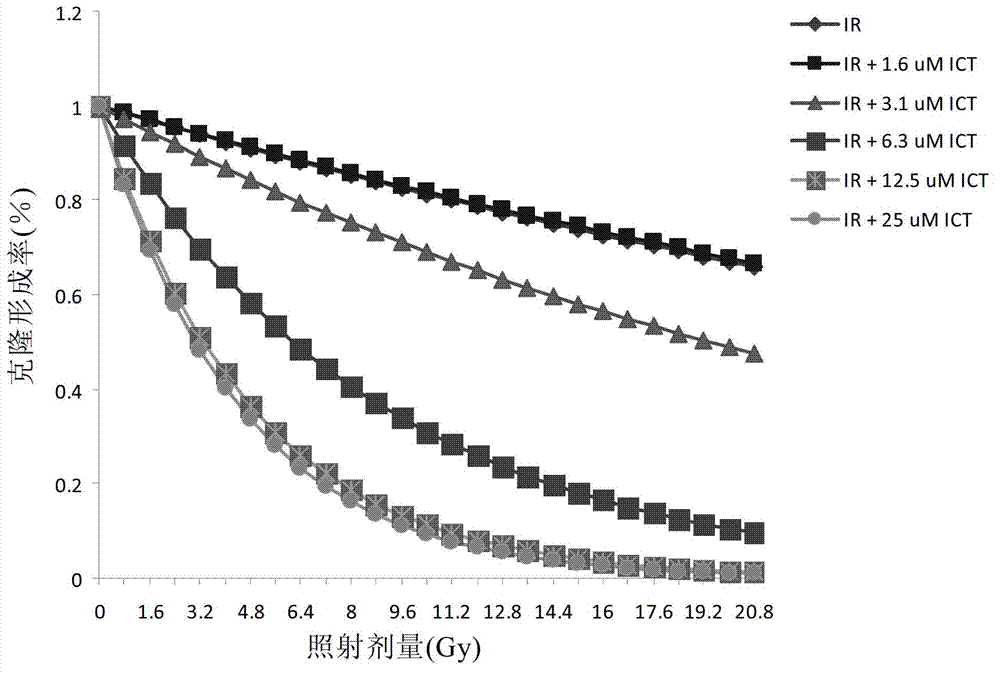
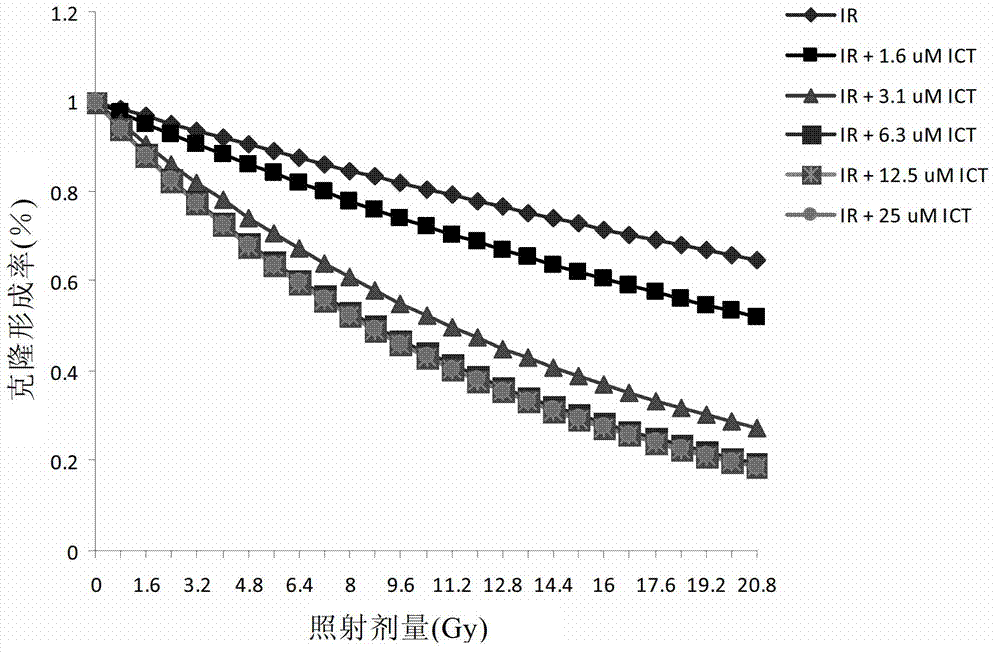
Preparation of icaritin and derivatives thereof and application of icaritin and derivatives of icaritin in radiotherapy
A technology of icariin and its derivatives, which is applied in the preparation of icariin or its derivatives and its new medical application, and the preparation of drugs that increase the effect of radiation therapy and reduce the side effects of radiation therapy. Icaritin or its derivatives In the field of drug preparation, it can solve the problems of reducing the side effects of radiotherapy and increasing the effect of radiotherapy with icariin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0183] Embodiment 1, the preparation of icariin derivative ICT-5-Fu condensate:
[0184]
[0185] 30 ml of tetrahydrofuran, 1 mmol of raw material ICT, 1 mmol of 5-fluorouracil (5-Fu), 1.3 mmol of DEAD and 1.3 mmol of triphenylphosphine were sequentially added into a 100 ml three-neck flask. The reaction was stirred overnight at room temperature. After the reaction was completed, 20 ml of water was added to the reaction system, extracted with ethyl acetate, the organic phases were combined, dried, filtered, concentrated to obtain a crude product, and purified by silica gel column chromatography to obtain pure ICT-5-Fu with a yield of 67%.
Embodiment 2
[0186] Embodiment 2, icariin derivative ICDE-01~ICDE-03 (when R 1 is methyl, R 2 for time) preparation:
[0187] 2.1 Preparation of intermediate IC-02:
[0188] Among them, R 1 For -Me:
[0189] Add 50 ml of acetone to a 100 ml three-necked flask, then, under stirring conditions, add 1 mmol of raw material IC-01, 1.2 mmol of allyl bromide and 1.5mmolK 2 CO 3 , the reaction solution was heated to reflux and stirred for 10 hours. After the reaction was completed, the solvent was evaporated to dryness under reduced pressure and recrystallized from ethanol to obtain the product IC-02 as a yellow solid with a yield of 70%.
[0190] 2.2 Preparation of intermediate IC-03:
[0191] Among them, R 1 For-Me, R 2 for Time:
[0192]In the there-necked flask of 100 milliliters, add 50 milliliters of methanol, then, under stirring condition, add 1mmol raw material IC-02 successively, 1.2mmol aldehyde (R 2 CHO) and 1.5mmol sodium ethylate, the reaction solution was heated...
Embodiment 3
[0205] Embodiment 3, icariin derivative ICDE-01 (when R 1 is benzyl, R 2 for time) preparation:
[0206] Among them, R 1 is benzyl, R 2 for Time:
[0207] Add 50 ml of methanol into a 100 ml three-neck flask, then exchange the reaction system with nitrogen three times, add 1 mmol of raw material IC-04, 10 mg of 10% Pd / C in sequence, and stir for 10 hours under hydrogen atmosphere. After the reaction was completed, the solvent was evaporated to dryness under reduced pressure, and recrystallized from ethanol / ethyl acetate to obtain the product ICDE-01 as a yellow solid with a yield of 60%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com