Use of recombinant adamts13 in the preparation of intracerebral hemorrhage drugs
A technology of uses and drugs, applied in the field of biopharmaceuticals, can solve the problems that have not yet been seen, and achieve the effect of increasing the safety of thrombolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] 1. Materials and methods
[0109] (1) Reagents and antibodies
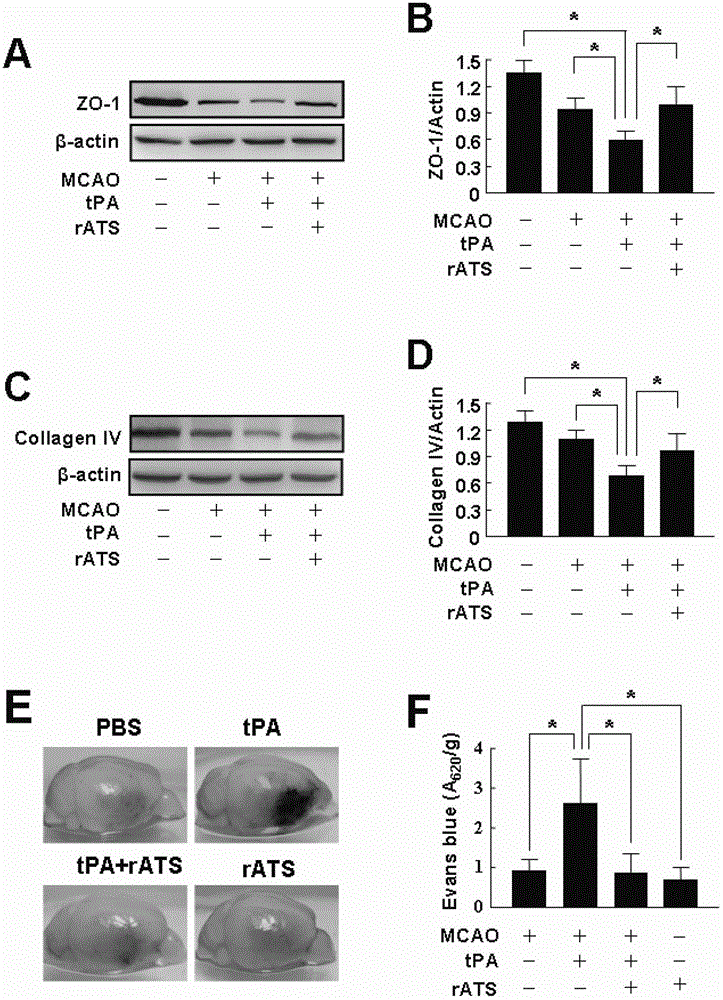
[0110] Evans blue, Drabkin, paraformaldehyde, D-glucose, formamide, wortmannin (purchased from Sigma-Aldrich) (St Louis, MO, USA), human recombinant tPA (Aitone) was purchased from Boehringer Ingelheim (Mannheim, Germany), human VWF protein was purchased from Haematologic Tecnologies (Essex Junction, VT, USA), human recombinant ADAMTS13 was purchased from R&D systems (neapolis, MN, USA), and fasudil was purchased from Calbiochem (San Diego, CA, USA). Primary antibodies include rabbit anti-zonula occludens (ZO-1) (Invitrogen, Camarillo, CA, USA), rabbit anti-collagen IV (Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-VEGF (vascular endothelial growth factor) ) (Abcam, MA, USA), goat anti-Angiopoietin(Ang)-1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), goat anti-Ang-2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-phospho-Akt (serine 473) (Cell Signaling Tecnology, Beverly, MA...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com