Cell freezing medium
A technology for cryopreservation and cells, which is applied in the field of cell therapy and can solve the problems of introduction of pollution and poor cell activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1, the comparison of three kinds of different cell cryopreservation liquids
[0018] 1. Experimental Design
[0019] Taking the commonly used cell freezing solution in the laboratory (fetal bovine serum+DMSO, hereinafter referred to as the control freezing solution A; see Situ Zhenqiang et al. "Cell Culture") as a contrast, compare the cell freezing solution developed by the inventors (cell freezing Preservation solution C) and umbilical cord blood hematopoietic stem cells commonly used cryopreservation solution (Dextran-40+DMSO, hereinafter referred to as umbilical cord blood cryopreservation solution B; Rubinstein et al., Proc.Nati.Acad.Sci.USA, Vol.92, pp.10119 -10122, Oct1995) for cryopreservation performance of twice subcultured lymphocytes.
[0020] 2. Experimental method
[0021] 2.1 Isolation and inoculation of peripheral blood mononuclear cells
[0022] Peripheral blood was collected from 3 healthy donors, 45 mL / person, and peripheral blood mononu...
Embodiment 2
[0044] Embodiment 2, to the optimization of cell cryopreservation liquid formula
[0045] 1 Experimental design
[0046] Adjust the composition of each component in the cryopreservation solution C formula in Example 1 (Table 3 below), and find the optimal concentration ratio through experimental comparison. Experimental method is the same as embodiment 1.
[0047]Table 3 optimizes the cryopreservation solution C
[0048] group
20%HSA(v / v)
DMSO (v / v)
Dextran 40 Glucose Injection (v / v)
1
10%
5%
85%
2
20%
5%
75%
3
30%
5%
65%
4
40%
5%
55%
[0049] 2 Experimental results
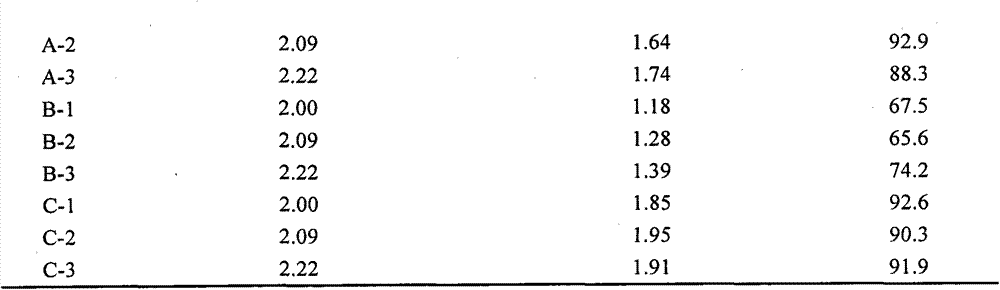
[0050] The experimental results are shown in Table 4 below. Wherein the second group of experimental results (i.e. the cryopreservation solution C in Example 1) is the best, but other groups are also better than cord blood cryopreservation solution B.
[0051] Table 4. Experimental results of modified cryo...
Embodiment 3
[0053] Embodiment 3, to the cryopreservation effect of the cell of different concentration
[0054] 1. Experimental Design
[0055] The cryopreservation solution C prepared in Example 1 was used to freeze cells with different concentrations, and the number and activity of cells after thawing were detected, and the applicable cell concentration range of the cryopreservation solution C was studied.
[0056] 2. Experimental method
[0057] Operations such as cell subculture, recovery after cryopreservation, etc. were as in Example 1. Prepare 50 ml of cell freezing solution according to the formula of freezing solution C in Example 1. Take the cell culture bottle out of the cell culture incubator, pat and mix the cell suspension in the bottle, and use a pipette to draw 0.5 mL for cell counting. According to the counting results, divide into three cell suspensions, and refer to the following table for the number of cells in each portion:
[0058] Table 5. Different cell freezin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com