4-methoxy-n,n-bisubstituted phenyl-1,3-benzene bisulfonamide compound and application thereof
A technology of benzenedisulfonamide and dimethylphenyl, applied in the field of anti-platelet aggregation drugs, can solve unseen problems and the like, and achieve the effects of simple preparation method, strong anti-platelet aggregation activity and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] A 4-methoxy-N,N'-bis(4-fluorobenzyl)-1,3-benzenedisulfonamide (C 21 N 2 o 5 S 2 f 2 h 20 ) preparation method, the steps are as follows:
[0017] 1) Add 0.63g (5mmol) p-fluorobenzylamine and 0.75g (2.5mmol) 4-methoxy-1,3-benzenedisulfonyl chloride into 10ml ethyl acetate and mix well, react at 0°C for 24h , and the solvent was distilled off to obtain the target crude product;
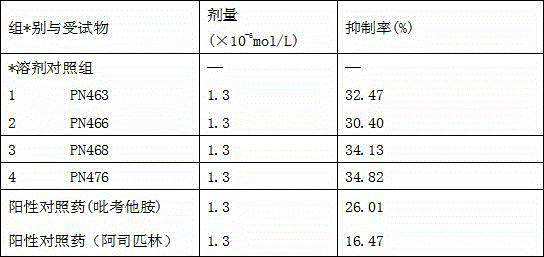
[0018] 2) Put the above target crude product into a single-necked bottle after drying, add 25ml of acetone, heat to boiling, cool to room temperature, filter and stand still, and then the pure target product can be obtained. After recrystallization with acetone, 0.98 g of transparent target crystals were obtained. Yield: 82.7%, mp: 164.5-166.8C. The group number of the compound in the rabbit platelet aggregation reaction experiment is PN463.
[0019] Infrared spectroscopy (IR) and nuclear magnetic resonance spectroscopy ( 1 H-NMR) confirmed the structure of PN463: IR (KBr, cm -1 ):3267...
Embodiment 2
[0021] A 4-methoxy-N,N'-bis(2-fluorobenzyl)-1,3-benzenedisulfonamide (C 21 N 2 o 5 S 2 f 2 h 20 ) preparation method, the steps are as follows:
[0022] 1) Add 0.63g (5mmol) o-fluorobenzylamine and 0.75g (2.5mmol) 4-methoxy-1,3-benzenedisulfonyl chloride into 10ml dichloromethane and mix well, react at 0°C for 30h , and the solvent was distilled off to obtain the target crude product;
[0023] 2) Put the above target crude product into a single-necked bottle after drying, add 30ml of absolute ethanol, heat to boiling, cool to room temperature, filter and stand still, and the pure target product can be obtained. After recrystallization from absolute ethanol, 0.8 g of white target crystals were obtained. Yield: 65.89%, mp: 169.1-171.2 o c. The group number of the compound in the rabbit platelet aggregation reaction experiment is PN466.
[0024] Infrared spectroscopy (IR) and nuclear magnetic resonance spectroscopy ( 1 H-NMR) confirmed the structure of PN466: IR (KBr, ...
Embodiment 3
[0026] A 4-methoxy-N,N'-bis(4-fluorophenethyl)-1,3-benzenedisulfonamide (C 23 N 2 o 5 S 2 f 2 h 24 )) preparation method, the steps are as follows:
[0027] 1) Add 0.7g (5mmol) p-fluorophenethylamine and 0.75g (2.5mmol) 4-methoxy-1,3-benzenedisulfonyl chloride into 10ml acetone and mix well, react at 0°C for 24h, The solvent is distilled off to obtain the target crude product;
[0028] 2) Put the above target crude product into a one-necked bottle after drying, add 20ml of methanol, heat to boiling, cool to room temperature, filter and stand still, and then the pure target product can be obtained. After recrystallization from methanol, 0.65 g of white target crystals were obtained. Yield: 52.0%, mp: 151.6-153.1 o c. The group number of the compound in the rabbit platelet aggregation reaction experiment is PN468.
[0029] Infrared spectroscopy (IR) and nuclear magnetic resonance spectroscopy ( 1 H-NMR) confirmed the structure of PN468: IR (KBr) cm -1: 3190.62, 1605....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com