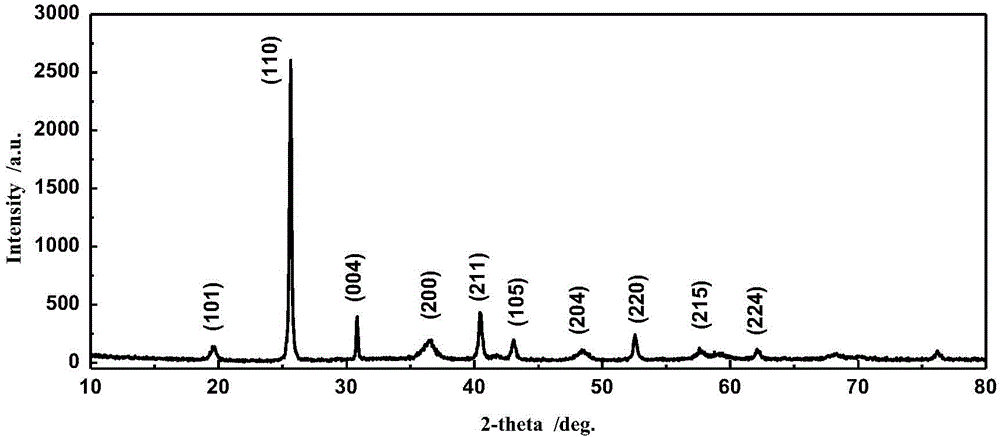
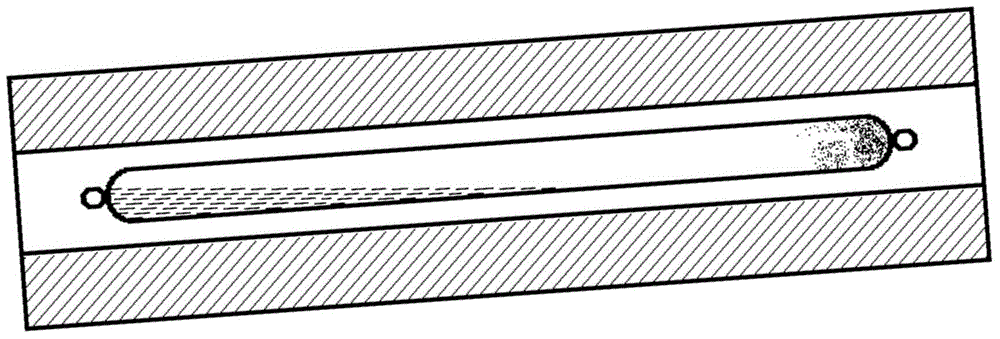
Method for synthesizing mercurous iodide
A technology of mercurous iodide and mercuric iodide, applied in directions such as mercuric halide, can solve the problems of difficulty in preparing raw materials of mercurous iodide, and achieve the effect of wide crystallization temperature range and easy control.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The method for the synthesis of mercurous iodide in the present embodiment may further comprise the steps:
[0034] S1: Clean the ampoule and dry it for later use;
[0035] S2: Measure mercury and mercury iodide as the reaction raw materials, and put the measured mercury and mercury iodide into the ampoule prepared in S1, and vacuum the ampoule until the vacuum degree reaches 1×10 -2 Seal the ampoule at Pa;
[0036] S3: Fix the ampoule in S2 vertically in the reaction furnace, and raise the temperature of the reaction furnace to 300°C at a heating rate of 20°C / h;
[0037] S4: After all the mercury iodide is melted, let stand for 4 hours;
[0038] S5: Lower the reaction furnace at a cooling rate of 200°C / h to any temperature below the medromic reaction temperature of the iodine-mercury binary system and above the eutectic reaction temperature, keep warm for 2 hours and then cool to room temperature with the furnace;
[0039] S6: Take out the product obtained in the am...
Embodiment 2
[0045] The method for the synthesis of mercurous iodide in the present embodiment may further comprise the steps:
[0046] S1: Clean the ampoule and dry it for later use;
[0047] S2: Measure mercury and mercury iodide as the reaction raw materials, and put the measured mercury and mercury iodide into the ampoule prepared in S1, and vacuum the ampoule until the vacuum degree reaches 1×10 -3 Seal the ampoule at Pa;
[0048] S3: Fix the ampoule in S2 vertically in the reaction furnace, and raise the temperature of the reaction furnace to 370°C at a heating rate of 100°C / h;
[0049] S4: After all the mercury iodide is melted, let stand for 1 hour;
[0050] S5: Lower the reaction furnace at a cooling rate of 400°C / h to any temperature below the medromic reaction temperature of the iodine-mercury binary system and above the eutectic reaction temperature, keep it warm for 10 hours, and then cool to room temperature with the furnace;
[0051] S6: Take out the product obtained in t...
Embodiment 3
[0054] The main steps of the method for synthesizing mercurous iodide in this embodiment are the same as those in Examples 1 and 2, except that the reaction raw material mercuric iodide in this embodiment is formed from iodine and mercury in the synthetic process of mercurous iodide Synthetic, specifically, the synthetic mercurous iodide method in the present embodiment comprises the following steps:
[0055] S1: Clean the ampoule and dry it for later use;
[0056] S2: includes the following steps:
[0057] S21: Measure mercury and iodine as the raw materials for the reaction, put the measured mercury and iodine into the ampoule prepared in S1, and evacuate the ampoule until the vacuum degree reaches 1×10 -2 Seal the ampoule at Pa;
[0058] S22: Fix the ampoule in S21 in the tube furnace with two temperature zones and make the mercury and iodine contained in the ampoule located at the high temperature end of the tube furnace with two temperature zones; the structure of the t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com