Podophyllotoxin derivative as well as salt, preparation method and application thereof
A technology of podophyllotoxin and derivatives, applied in the field of podophyllotoxin derivatives, which can solve problems such as interference with DNA replication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
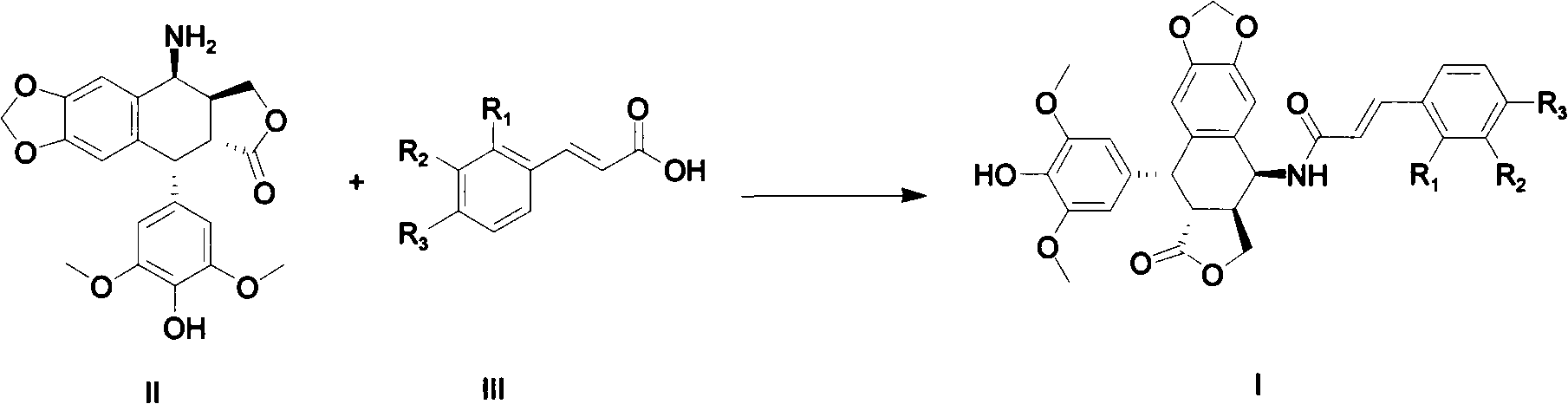
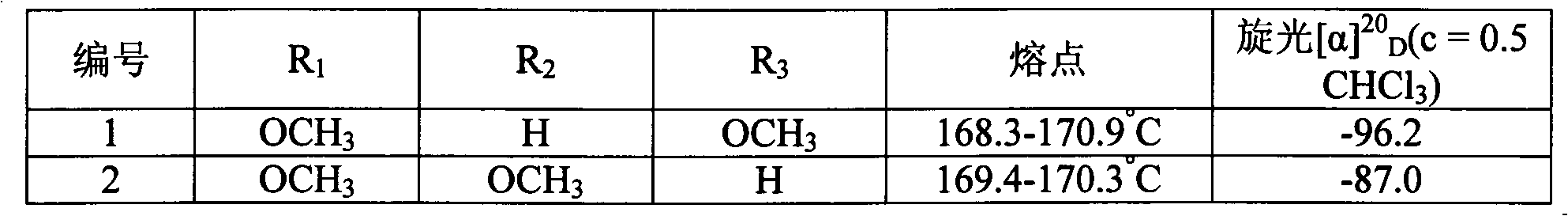
[0072] Example 1 Preparation of 4β-4-(2", 4"-dimethoxyphenylacrylamide)-4-deoxy-4'-norepipodophyllotoxin (1)
[0073] 2,4-dimethoxycinnamic acid (115mg, 0.55mmol), 1-ethyl-3-[3-(dimethylamino)propyl]carbodiimide hydrochloride (EDCI) (96mg, 0.5mmol), 1-hydroxybenzotriazole (70mg, 0.5mmol), were added into dichloromethane (10ml), under nitrogen protection, stirred at room temperature (10°C-25°C) for 30min, then added compound 4β-amino-4 '-O-norepipodophyllotoxin (200 mg) was added dropwise with 0.2 ml of N,N-diisopropylethylamine for reaction, TLC followed the reaction end point, and the reaction lasted for about three hours. The reaction was stopped, washed with 1M HCl acid, then washed with water, dried overnight with anhydrous sodium sulfate, filtered to remove anhydrous sodium sulfate, and the filtrate was evaporated to dryness under reduced pressure to obtain a white solid. In DCM / CH 3 OH was used for gradient elution with developing solvent (volume ratio: 40:1-10:1). The...
Embodiment 2
[0096] Example 2 Preparation of 4β-4-(2,3-dimethoxyphenylacrylamide)-4-deoxy-4'-norepipodophyllotoxin (2)
[0097] 115mg of 2,3-dimethoxycinnamic acid, 1-ethyl-3-[3-(dimethylamino)propyl]carbodiimide hydrochloride (EDCI) (96mg, 0.5mmol), 1 -Hydroxybenzotriazole (70mg, 0.5mmol), was added into dichloromethane (10ml), stirred for 30min, then compound 4β-amino-4'-O-norepipodophyllotoxin (200mg) was added dropwise 0.2ml N, N-diisopropylethylamine, react at room temperature. Post-treatment process: the reaction solution was first acid-washed with 1M HCl, then washed with water, and dried overnight with anhydrous sodium sulfate. Anhydrous sodium sulfate was removed by filtration, and the filtrate was evaporated to dryness, purified by column chromatography, and purified with DCM / CH 3 OH (volume ratio: 40:1 ~ 10:1) was used for gradient elution of the developing solvent to obtain the pure product. The post-treatment of the rest of the compounds is the same as this method. 220 mg ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com