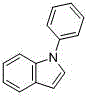
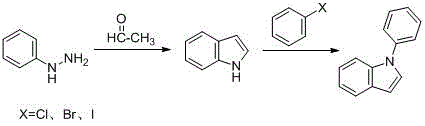
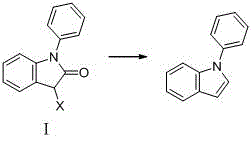
A kind of method for preparing 1-phenylindole
A technology of phenylindole and phenylindolinone, which is applied in the field of synthesizing 1-phenylindole, a key intermediate of iminostilbene, and synthesizing 1-phenylindole, can solve the need for column chromatography, original Expensive excipients, unsafe production factors, etc., to achieve the effect of optimizing reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0026] (1) Add 20.9 g (0.1 mol) of N-phenylindolinone and 200 mL of cyclohexane into a four-neck flask, stir, and heat to reflux.
[0027] (2) Add 10.78g (0.2mol) of potassium borohydride and 1.33g (0.01mol) of aluminum chloride in batches, react for 11-24 hours, and track the reaction by TLC. After the reaction, cool slightly, filter, add an appropriate amount of water to the organic layer to quench, filter to obtain the filtrate, separate the liquids to obtain the organic layer, dry over anhydrous magnesium sulfate, filter, and recover the solvent by rotary evaporation under reduced pressure to obtain 12.54 g of 1-phenylindole , yield 64.97%.
example 2
[0029] (1) Add 20.9g (0.1mol) of N-phenylindolinone and 400mL of toluene into a four-neck flask, stir and heat to reflux.
[0030] (2) Add 11.4g (0.3mol) of sodium borohydride and 11.1g (0.1mol) of calcium chloride in batches, react for 11-24 hours, and track the reaction by TLC. After the reaction, cool slightly, filter, quench the organic layer with an appropriate amount of water, filter to obtain the filtrate, separate the liquids to obtain the organic layer, dry over anhydrous magnesium sulfate, filter, and recover the solvent by rotary evaporation under reduced pressure to obtain 17.17g of 1-phenylindole , yield 88.96%.
example 3
[0032] (1) Add 20.9g (0.1mol) of N-phenylindolinone and 600mL of tetrahydrofuran into a four-necked flask, stir, and heat to reflux.
[0033] (2) Add 19g (0.5mol) of sodium borohydride and 13.6g (0.1mol) of zinc chloride in batches, react for 11-24 hours, and track the reaction by TLC. After the reaction, cool slightly, filter, quench the organic layer with an appropriate amount of water, filter to obtain the filtrate, separate the liquids to obtain the organic layer, dry over anhydrous magnesium sulfate, filter, and recover the solvent by rotary evaporation under reduced pressure to obtain 18.49 g of 1-phenylindole , yield 95.80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com