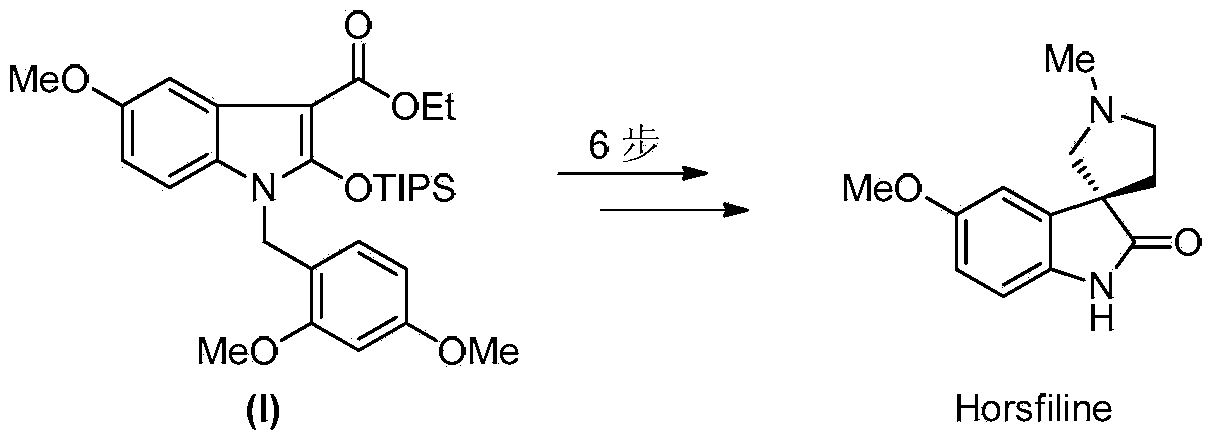
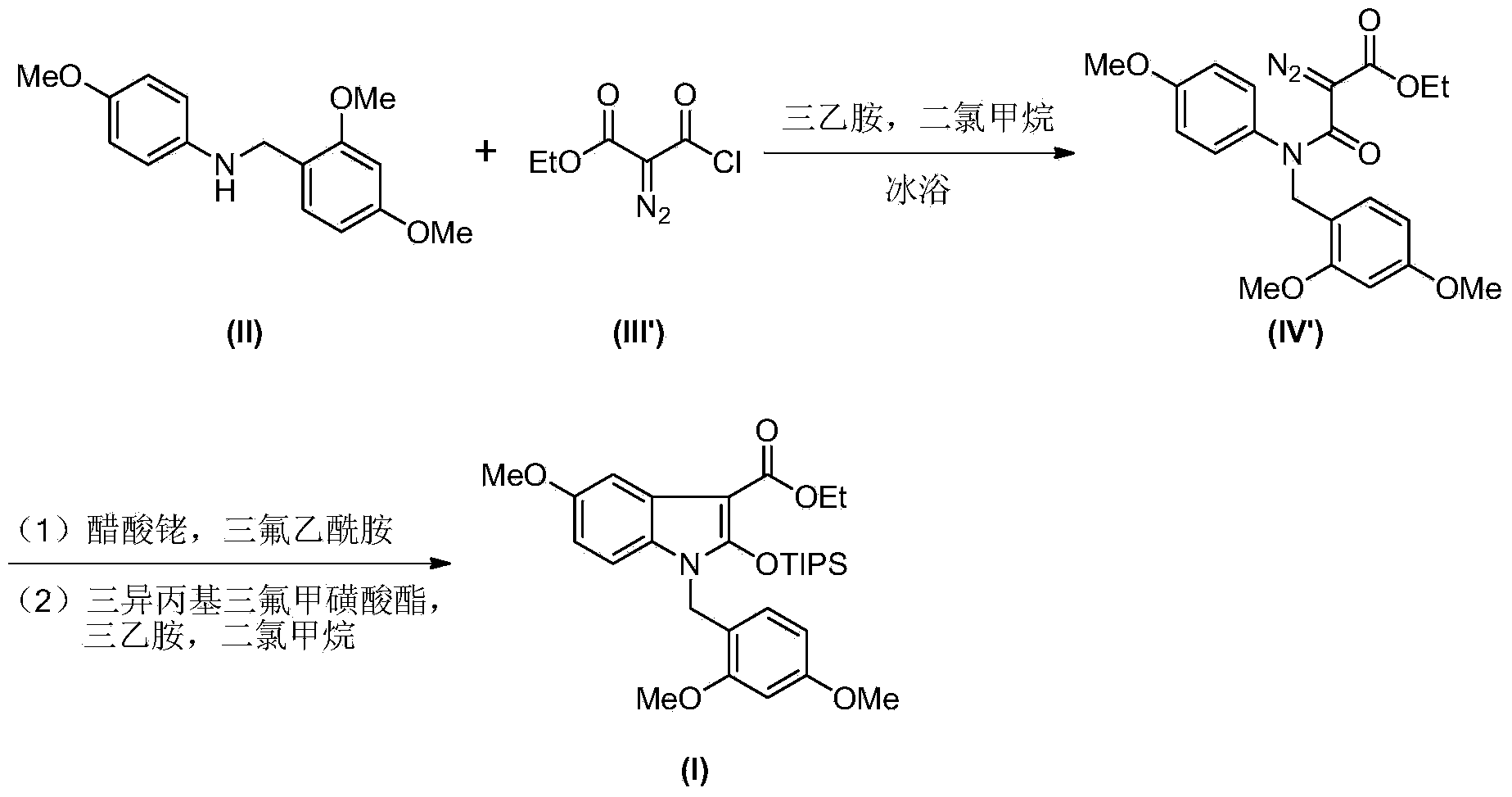
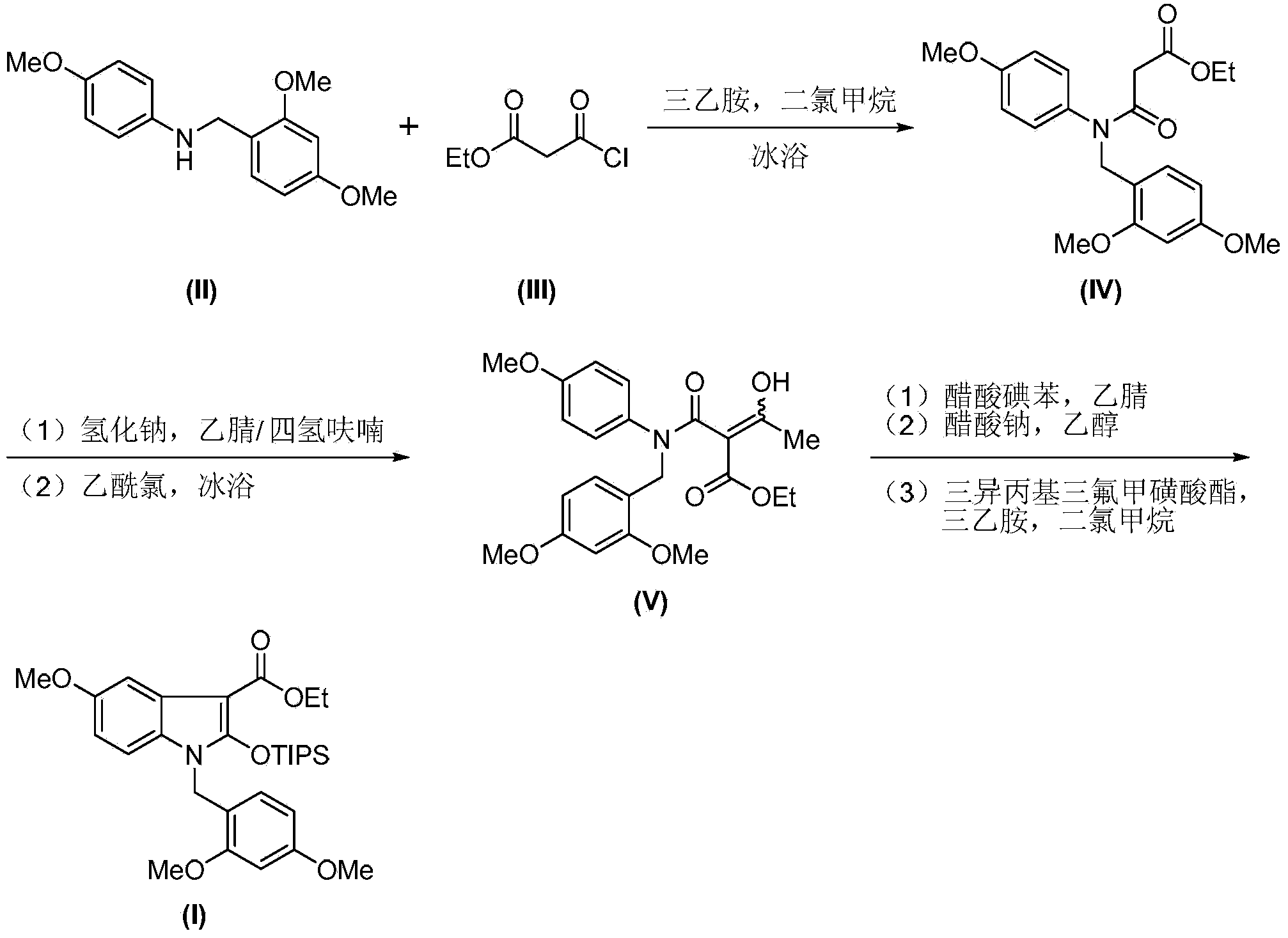
Synthesis method of 2-indolone-3-carboxylate derivatives
A synthesis method and derivative technology, applied in the field of synthesis of 2-indolinone-3-carboxylate derivatives, can solve the problems of potential environmental hazards and high commercial prices, and achieve environmental friendliness, simple operation, The effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Monoethyl malonate amide derivative (ethyl 3-oxo-3-[(2,4-dimethoxybenzyl)(4-methoxyphenyl)amino]propionate) (IV) preparation
[0024] Dissolve monoethylmalonyl chloride (III) (2.6g, 17.6mmol) in anhydrous dichloromethane (130mL), cool the resulting solution to 0°C, and add substituted aniline (N-(2 , 4-dimethoxybenzyl)-4-methoxyaniline) (II) (4.0g, 16.6mmol), stirred until dissolved, slowly added triethylamine (2.5 mL), after the dropwise addition was completed, the stirring was continued for 30 minutes, and the reaction was completed. Add saturated ammonium chloride solution (100mL) to the reaction solution, place the mixture in a separatory funnel to collect the organic layer, wash with water (150mL), dry the organic phase over anhydrous sodium sulfate and concentrate under reduced pressure to obtain the crude product , the crude product was separated and purified by column chromatography to obtain 5.5 g of light yellow oily liquid, yield: 85%.
[0025] 1 H NMR (4...
Embodiment 2
[0028] Enol compound (ethyl 2-[(2,4-dimethoxybenzyl)(4-methoxyphenyl)carbamoyl]-3-hydroxy-2-butenoate) (V) preparation
[0029] Suspend sodium hydride (524 mg, 13 mmol) in a mixed solution of anhydrous tetrahydrofuran / acetonitrile (33 mL, V THF :V MeCN =1:1) and stir evenly, add 3-oxo-3-[(2,4-dimethoxybenzyl)(4-methoxyphenyl) dropwise to the mixture at 0°C Ethyl amino]propionate (IV) (1.5 g, 3.95 mmol) in dry THF / acetonitrile (33 mL, V THF :V MeCN =1:1), continue to stir for 30 minutes after the dropwise addition. Acetyl chloride (340uL) was slowly added dropwise to the obtained mixture at 0°C, and the obtained mixture was protected with nitrogen, stirred for 6 hours and gradually warmed to room temperature. After the completion of the reaction monitored by TLC, ethyl acetate (100 mL) was added to the reaction system and transferred to a separatory funnel, washed with water (100 mL), and the organic phase was dried over anhydrous sodium sulfate and concentrated under redu...
Embodiment 3
[0033] 2-indolinone-3-carboxylate derivatives (1-(2,4-dimethoxybenzyl)-5-methoxy-2-[(triisopropylsilyl)oxy] -1-H-indole-3-carboxylic acid ethyl ester) (I)
[0034] 2-[(2,4-dimethoxybenzyl)(4-methoxyphenyl)carbamoyl]-3-hydroxy-2-butenoic acid ethyl ester (V) (622mg, 1.45mmol) Dissolve in acetonitrile (30 mL), add iodobenzene acetate powder (562 mg, 1.75 mmol) to the resulting solution at one time, stir and react at room temperature for 30 minutes, until the disappearance of the raw material as monitored by TLC. Add ethanol (5 mL) and sodium acetate powder (2.4 g, 29 mmol) to the reaction solution, and stir at room temperature for 48 hours. The solvent was removed under reduced pressure, ethyl acetate (50 mL) was added to the reaction system and transferred to a separatory funnel, washed with water (50 mL), the organic phase was dried over anhydrous sodium sulfate and concentrated under reduced pressure to obtain a crude product. The crude product was dissolved in anhydrous di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com