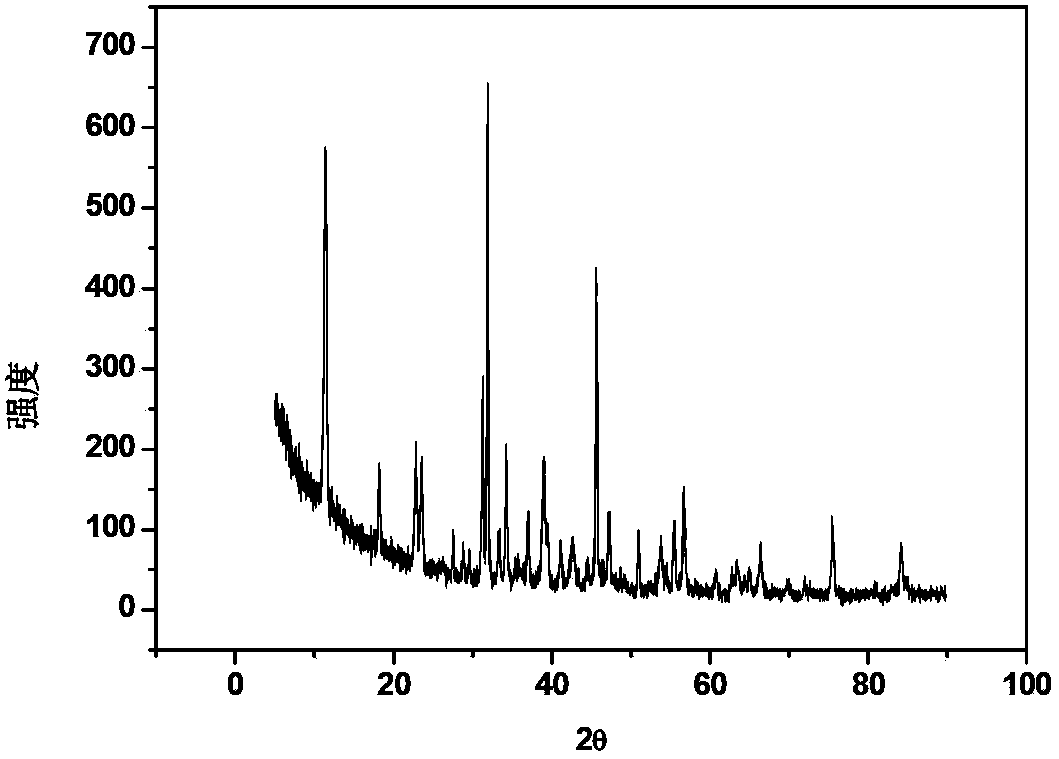
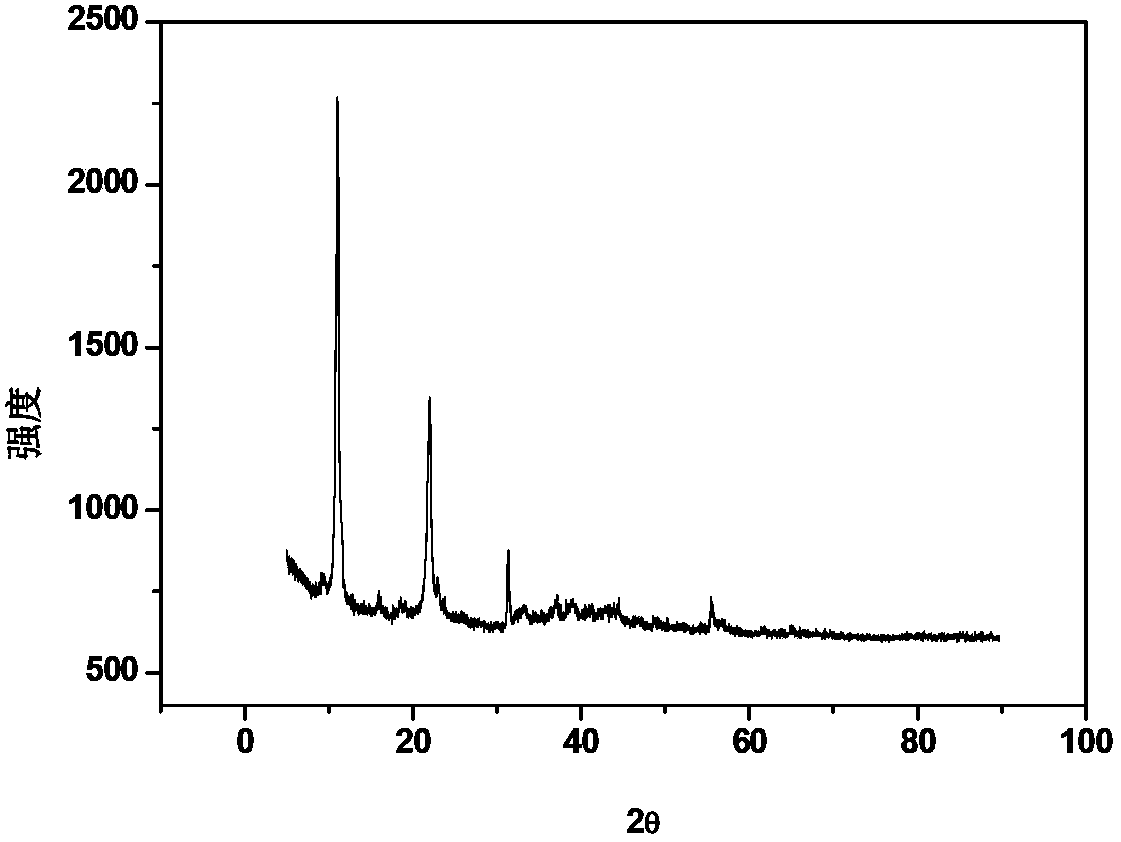
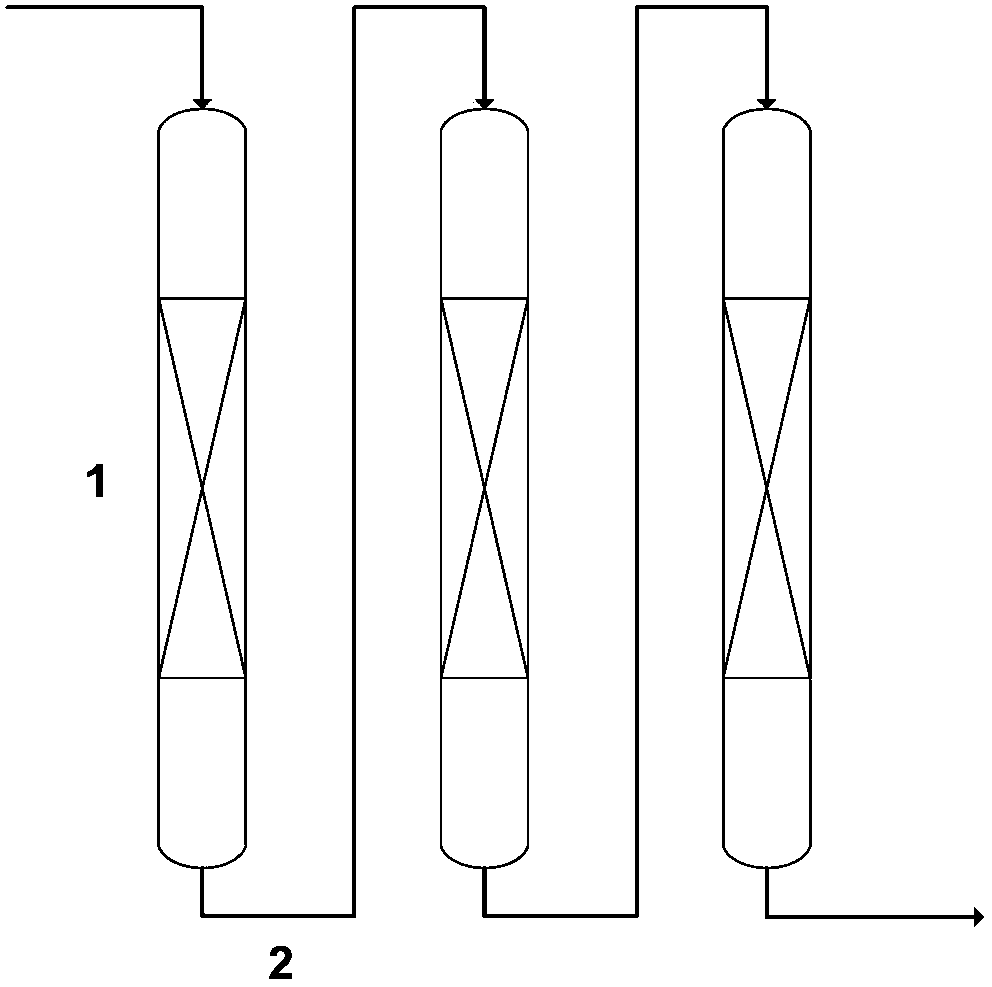
Method for removing harmful anions from aqueous solution by adopting Friedel salt or Kuzel salt
An aqueous solution and anion technology, applied in the field of water treatment, can solve the problems of poor chemical stability, low mechanical strength, and low removal efficiency, and achieve the effects of easy operation, simple equipment, and large ion exchange capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] (1) Calcium chloride reagent is dissolved in water to make solution A with a concentration of 0.5mol / L;
[0045] (2) Add sodium hydroxide and aluminum hydroxide to water, heat to dissolve the sodium hydroxide and aluminum hydroxide, and obtain sodium aluminate solution B, in which the concentration of aluminum ions is 0.5mol / L, and Na in the sodium aluminate solution + with Al 3+ The molar ratio is 3.0;
[0046] (3) Add 300ml of solution B prepared in step (2) into the jacketed reaction tank, pass a circulating water bath into the jacket of the jacketed reaction tank, and adjust the temperature of solution B to 30°C, At a stirring speed of 300rpm, add 300ml of the solution A prepared in step (2) into the reaction tank, and after the addition, filter to obtain Friedel salt;
[0047] (4) washing the crude Friedel salt obtained after filtering in step (3) with water, and drying to obtain dried Friedel salt;
[0048] (5) Put the dried Friedel salt obtained in step (4) in...
Embodiment 2
[0051] (1) Dissolve calcium chloride, a by-product in the soda ash industry, in water to prepare solution A with a concentration of 0.8mol / L;
[0052] (2) Add sodium hydroxide and aluminum chloride to water, heat to dissolve sodium hydroxide and aluminum chloride, and obtain sodium aluminate solution B, in which the concentration of aluminum ions is 0.4mol / L, and Na in sodium aluminate solution + with Al 3+ The molar ratio is 6.0;
[0053] (3) Add 400ml of the solution B prepared in step (2) into the jacketed reaction tank, pass a circulating water bath into the jacket of the jacketed reaction tank, and adjust the temperature of solution B to 50°C. At a stirring speed of 400rpm, add 400ml of the solution A prepared in step (2) into the reaction tank, and after the addition is completed, filter to obtain Friedel salt;
[0054] (4) washing the crude Friedel salt obtained after filtering in step (3) with water, and drying to obtain dried Friedel salt;
[0055] (5) Pack the dri...
Embodiment 3
[0060] (1) Dissolve calcium chloride, a by-product in the soda ash industry, in water to prepare solution A with a concentration of 2.0mol / L;
[0061] (2) Add sodium hydroxide and aluminum sulfate to water, heat to dissolve sodium hydroxide and aluminum sulfate, and obtain sodium aluminate solution B, in which the concentration of aluminum ions is 1.2mol / L, and Na in sodium aluminate solution + with Al 3+ The molar ratio is 7.0;
[0062] (3) Add 380ml of the solution B prepared in step (2) into the jacketed reaction tank, pass a circulating water bath into the jacket of the jacketed reaction tank, and adjust the temperature of solution B to 70°C, At a stirring speed of 250rpm, add 380ml of the solution A prepared in step (2) into the reaction tank, and after the addition, filter to obtain Friedel's salt;
[0063] (4) washing the crude Friedel salt obtained after filtering in step (3) with water, and drying to obtain dried Friedel salt;
[0064] (5) Pack the dried Friedel sa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com