Dialdehyde-built nitrogen-containing or oxygen-containing heterocyclic compound with insecticidal activity and preparation method thereof
A compound and composition technology, applied in the field of new neonicotinoid insecticides, can solve the problems of narrow insecticidal spectrum, limited drug selectivity, restricted compound development, etc., so as to solve the problem of resistance and expand the insecticidal spectrum. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0061] The preparation method of the compound of the present invention
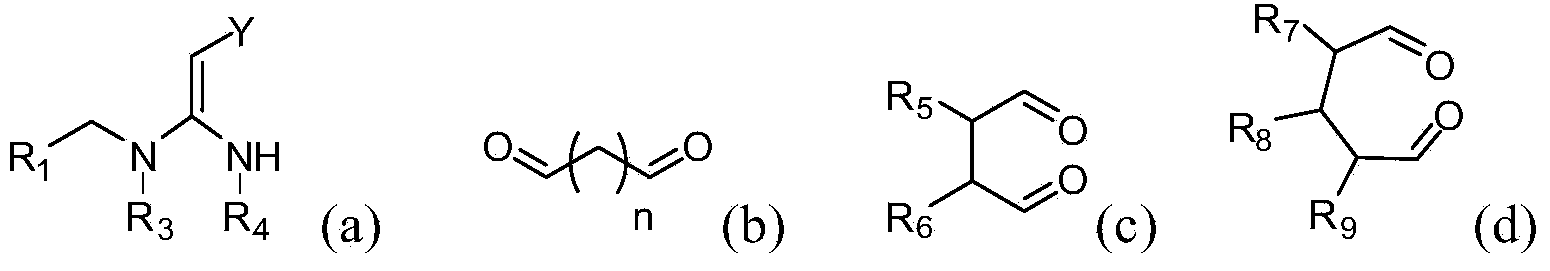
[0062] The compounds of the present invention can be synthesized by the reaction steps described above. Those skilled in the art can synthesize the compound of formula (a) in the reaction step according to the prior art documents, for example, refer to WO2006056108A1, WO2007101369A1 and PCT / CN2008 / 071115.
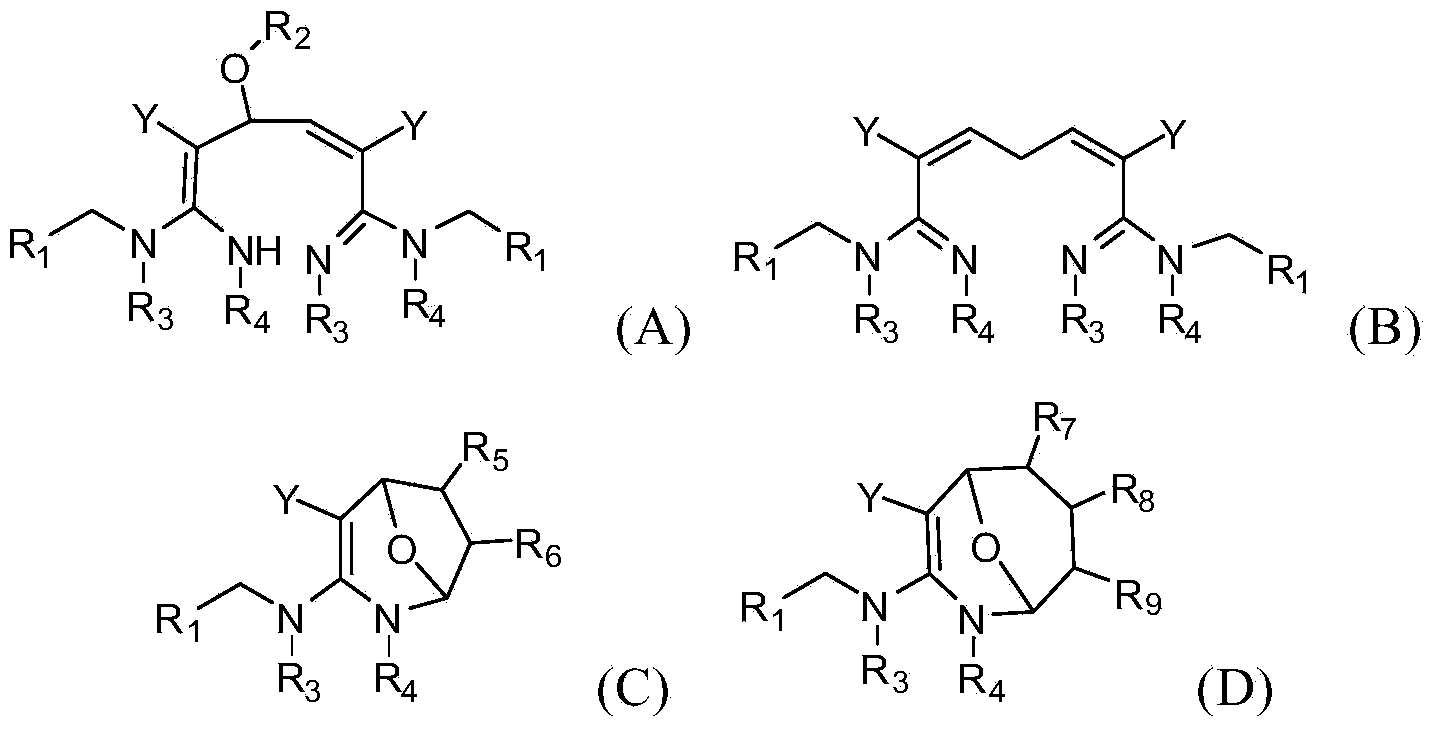
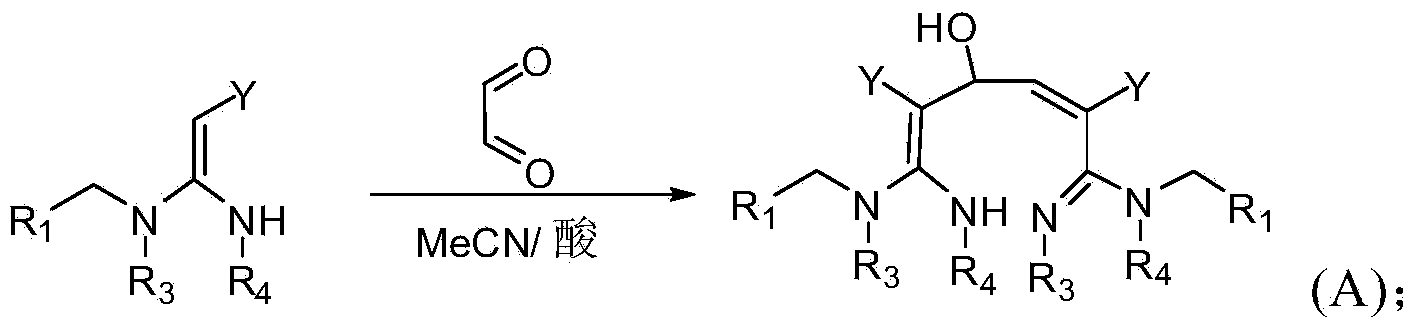
[0063] In a specific embodiment of the present invention, the synthetic method of formula (A) compound is as follows:
[0064]
[0065] In a specific embodiment of the present invention, the synthetic method of formula (B) compound is as follows:
[0066]
[0067] In a specific embodiment of the present invention, the synthetic method of formula (C) compound is as follows:
[0068]
[0069] In a specific embodiment of the present invention, the synthetic method of formula (C) compound is as follows:
[0070]
[0071] In one embodiment of the present invention, the compound of formula (...
Embodiment 1
[0105] Example 1 : 4-(1-((6-chloropyridin-3-yl)methyl)-4,5-dihydro-1H-imidazolidin-2-yl)-1-(1-((6-chloropyridine- Synthesis of 3-yl)methyl)imidazolidin-2-yl)-1,4-dinitro-3-buten-2-ol (compound 13)
[0106] Using 0.03mol of 2-chloro-5-chloromethylpyridine as a starting material, prepare 2-chloro-5-(2-nitromethylene-imidazolidin-1-ylmethanol according to the method described in WO2006056108A1 and WO2007101369A1 base)-pyridine, the yield was 56%; Rf = 0.46 (petroleum ether: ethyl acetate = 1:1); mp = 156.9°C-161.8°C. GC MS (m / s) 220(25), 126(100), 90(9).
[0107] 4-(1-((6-chloropyridin-3-yl)methyl)-4,5-dihydro-1H-imidazolidin-2-yl)-1-(1-((6-chloropyridine-3 -base) Synthesis of Methyl)imidazolidin-2-yl)-1,4-Dinitro-3-buten-2-ol
[0108]
[0109]Add 1.27g (0.005mol) of 2-chloro-5-(2-nitromethylene-imidazolidin-1-ylmethyl)-pyridine, 30ml of anhydrous acetonitrile, and 3ml of 30% glyoxal aqueous solution to 50ml In a round-bottomed flask, stir for half an hour, then add ...
Embodiment 2
[0111] Example 2 : 2-chloro-5-((-2-(-4-(1-((6-chloropyridin-3-yl)methyl)-4,5-dihydro-1H-imidazolidin-2-yl) Synthesis of -2-methoxy-1,4-dinitro-3-butenyl)imidazolidin-1-yl)methyl)pyridine (compound 14)
[0112]
[0113] Add 0.549g (0.001mol) of compound 1 into a 50ml round-bottomed flask, then add 10ml of methanol, 50ml of dichloromethane and a catalytic amount of concentrated hydrochloric acid, reflux, and track the reaction by TLC. After the reaction was completed, the solvent was removed, and the pure product was obtained as a yellow powder through column chromatography separation, with a yield of 62%.
[0114] mp=151.6-153.1℃; 1 H NMR (400Mz, DMSO-d 6 ):δ9.03(s,1H),8.38(d,J=2.0Hz,1H),8.36(d,J=2.0Hz,1H),7.81-7.85(m,2H),7.49-7.51(m, 2H),6.50(d,J=7.2Hz,1H),5.35(d,J=15.2Hz,1H),5.19(d,J=15.2Hz,1H),4.80(d,J 1 =7.2Hz,1H),4.77(d,J=16.8Hz,1H),4.69(d,J=16.8Hz,1H),3.68(s,3H),3.88-3.95(m,2H),3.61-3.85 (m,5H),3.38-3.41(m,1H)ppm; 13 C NMR (100Mz, DMSO-d 6 ): δ162.6, 158.7, 14...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com