Stable rabies virus humanized antibody combined reagent
A human antibody, rabies virus technology, applied in the direction of antiviral agents, antibodies, etc., can solve problems such as difficulties and achieve the effect of convenient use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Preparation of NM57 and NC08 antibody combination preparation.
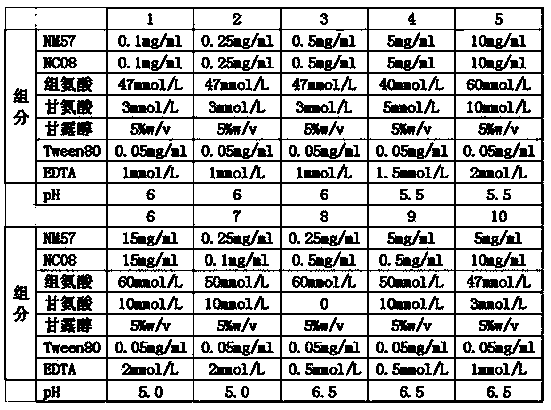
[0030] The liquid preparations of different antibody combination preparations were prepared according to the formulas in Table 1, and the solvent was pure water for injection to obtain semi-finished products.
[0031] Table 1. Formulas of different antibody combination preparations
[0032]
[0033] The semi-finished product is aseptically divided into vials, and the rubber stopper and aluminum-plastic cap are added to obtain the finished product.
Embodiment 2
[0034] Example 2 The 10 groups of samples numbered 1 to 10 prepared in Example 1 were stored according to the storage conditions in Table 2, and the neutralizing activity of the anti-rabies virus antibody in the solution preparation was regularly measured by rapid fluorescence focus inhibition test (RFFIT) , the product before preservation is used as a contrast, and the percentage value of the activity of the product after preservation and the activity of the product before preservation is defined as the activity of the product after preservation.
[0035] Table 2. Sample activity preserved under different conditions
[0036] Storage Conditions 0 weeks 1 week 1 37℃ 100% 105% 2 37℃ 100% 96% 3 37℃ 100% 110% 4 37℃ 100% 95% 5 37℃ 100% 95% 6 37℃ 100% 93% 7 37℃ 100% 98% 8 37℃ 100% 100% 9 ...
Embodiment 3
[0038] Example 3 The 10 groups of samples numbered 1 to 10 prepared in Example 1 were stored according to the storage conditions in Table 2, and were regularly analyzed by molecular sieve high-pressure liquid chromatography (SEC-HPLC, TSK G3000 SW XL 7.8mmI.D.x30cm) to detect the purity of the antibody in the solution preparation, and investigate the stability of the antibody NM57 and NC08 in the preparation.
[0039] Stability testing method:
[0040] Instrument: Waters HPLC system (photodiode array detector)
[0041] Column: TSK G3000 SW XL 7.8mmI.D.x30cm
[0042] Mobile phase: 200mmol / L PB, 1% isopropanol, pH7.0
[0043] Flow rate: 0.5ml / min
[0044] Loading amount: 100μg
[0045] Detection wavelength: 280nm
[0046] Table 3. Stability of samples preserved under different conditions
[0047] Example Storage Conditions 0 weeks 1 week 1 37℃ 100% 99.7% 2 37℃ 99.9% 99.7% 3 37℃ 100% 99.5% ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com