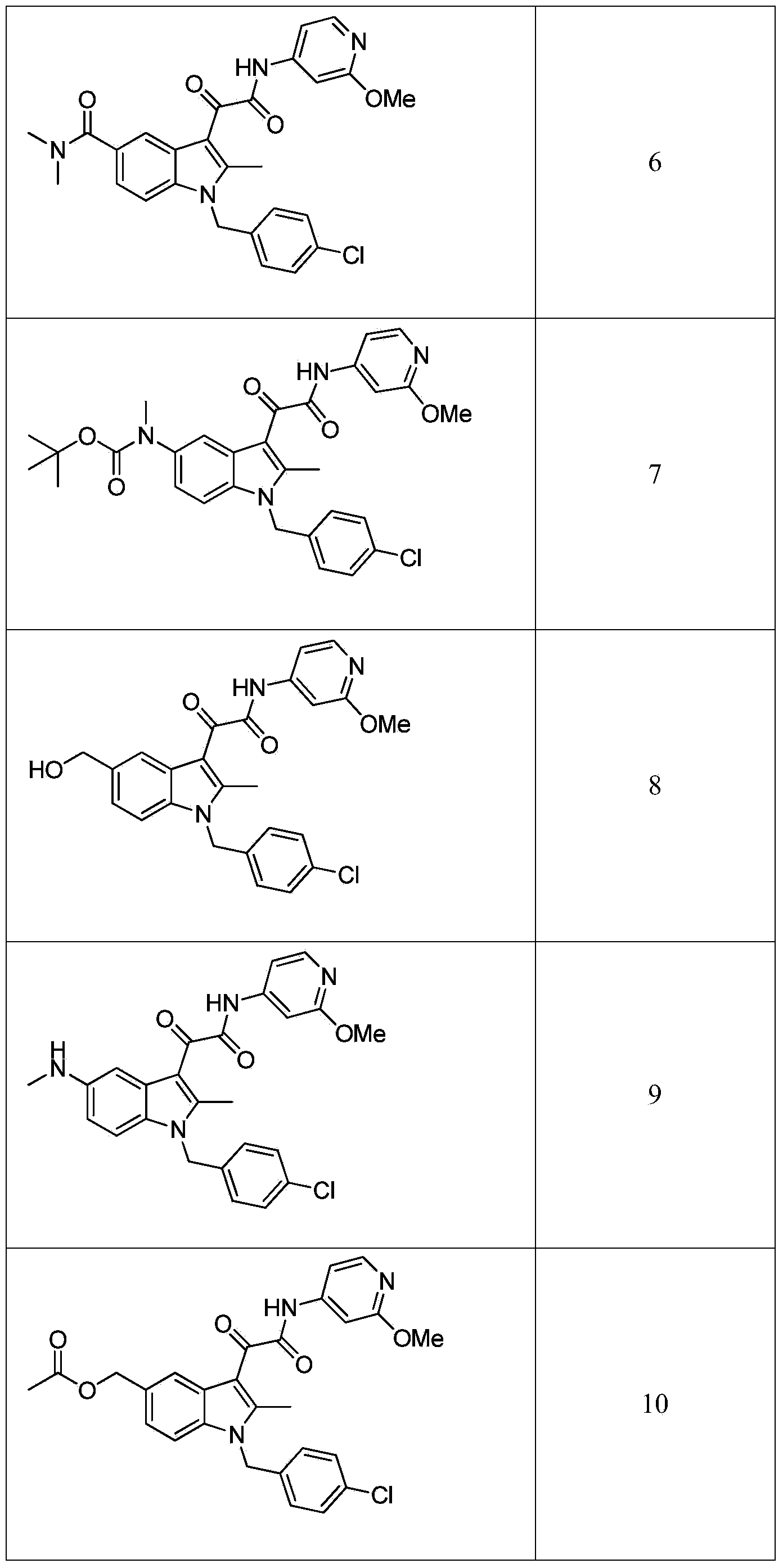
FAAH inhibitors
A technology of inhibitors and pharmaceuticals, applied in the field of indole and azaindole compounds, which can solve problems such as the increase of prostaglandin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0334] Embodiment 1 (see route 1, operation A, B, C and D)
[0335] 2-(1-((5-chloropyrazin-2-yl)methyl)-5-methoxy-2-methyl-1H-indol-3-yl)-N-(2-methoxy Pyridin-4-yl)-2-oxoacetamide (12)
[0336]
[0337] To a solution of 5-methoxy-2-methyl-1H-indole (3.45 g, 21.4 mmol) in dichloromethane (100 mL) was added oxalyl chloride (2.06 mL, 23.5 mmol) at 0°C. After 30 minutes, the reaction was allowed to warm to room temperature, and LCMS analysis indicated the presence of ketoacid chloride (via methanolysis product analysis). The reaction mixture was concentrated to dryness, then reconstituted in dichloromethane (100 mL) and cooled to 0 °C. Methanol (8.00 mL, 198 mmol) was added, then the reaction mixture was warmed to room temperature, causing a solid precipitate to form, which was filtered and washed with hexanes, dried to give 2-(5-methoxy- Methyl 2-methyl-lH-indol-3-yl)-2-oxoacetate (4.08 g, 16.5 mmol, 77% yield). The material was used without further purification. 1 HNMR...
Embodiment 2
[0341] Example 2 (see general route 2, operation E, F, C and D)
[0342] 2-(1-(4-chlorobenzyl)-2-methyl-1H-pyrrolo[3,2-b]pyridin-3-yl)-N-(2-methoxypyridin-4-yl) -2-oxoacetamide (3)
[0343]
[0344] To a solution of 2-methyl-1H-pyrrolo[3,2-b]pyridine (74.4 mg, 0.563 mmol) and 4-chlorobenzyl chloride (0.0780 mL, 0.619 mmol) in DMSO (8 mL) at room temperature Powdered potassium hydroxide (69.5mg, 1.24mmol) was added. The reaction was stirred at room temperature for 12 hours, then LCMS analysis indicated the reaction was complete. The reaction mixture was diluted in water and extracted with dichloromethane (3 x 50 mL), dried (sodium sulfate), filtered and concentrated to a clear residue. Purification was achieved by silica gel chromatography using 30 to 90% ethyl acetate in hexane over 80 minutes to afford 1-(4-chlorobenzyl)-2-methyl-1H-pyrrolo[3 ,2-b]pyridine (64.2 mg, 0.250 mmol, 44% yield). 1 H NMR (400MHz, CDCl 3 )δ (ppm): 8.41 (dd, 1H), 7.41 (d, 1H), 7.24 (d, 2H),...
Embodiment 3
[0347] Embodiment 3 (referring to general scheme 3, operation E and operation G)
[0348] 2-(1-(4-chlorobenzyl)-5-cyano-2-methyl-1H-indol-3-yl)-N-(2-methoxypyridine -4-yl)-2-oxoacetamide (15)
[0349]
[0350] To 2-methyl-1H-indole-5-carbonitrile (1.89g, 12.1mmol) and 1-chloro-4-(chloromethyl)benzene (1.53mL, 12.1mmol) in DMSO (40.3mL) at room temperature Powdered potassium hydroxide (1.39 g, 24.2 mmol) was added to the solution. The reaction mixture was stirred at room temperature for 12 hours, diluted in water, extracted with dichloromethane (3 x 50 mL), dried (sodium sulfate), filtered and concentrated to a clear residue. Purification was achieved by silica gel chromatography using 10 to 60% ethyl acetate in hexane over 75 minutes to give 1-(4-chlorobenzyl)-2-methyl-1H-indole-5 as an off-white solid -Formonitrile (2.75 g, 9.80 mmol, 81% yield). 1 H NMR (400MHz, CDCl 3 )δ(ppm):7.88(d,1H),7.34(dd,1H),7.26(d,2H),7.20(d,1H),6.86(d,2H),6.41(s,1H),5.29( s,2H), 2.30(...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com