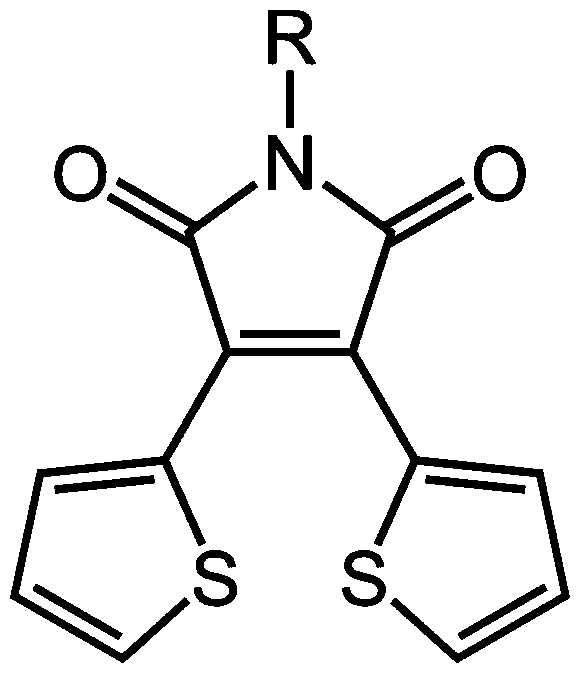
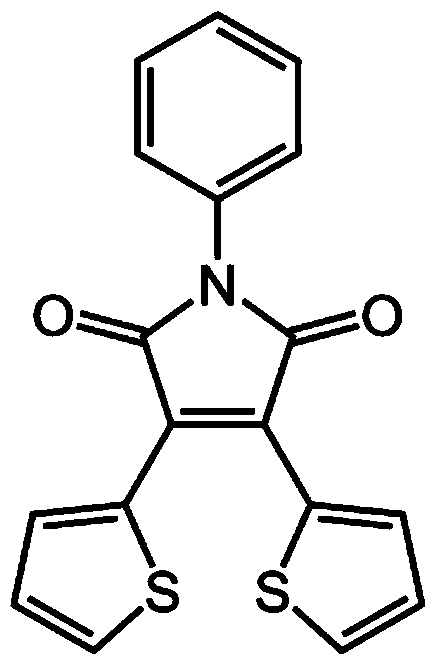
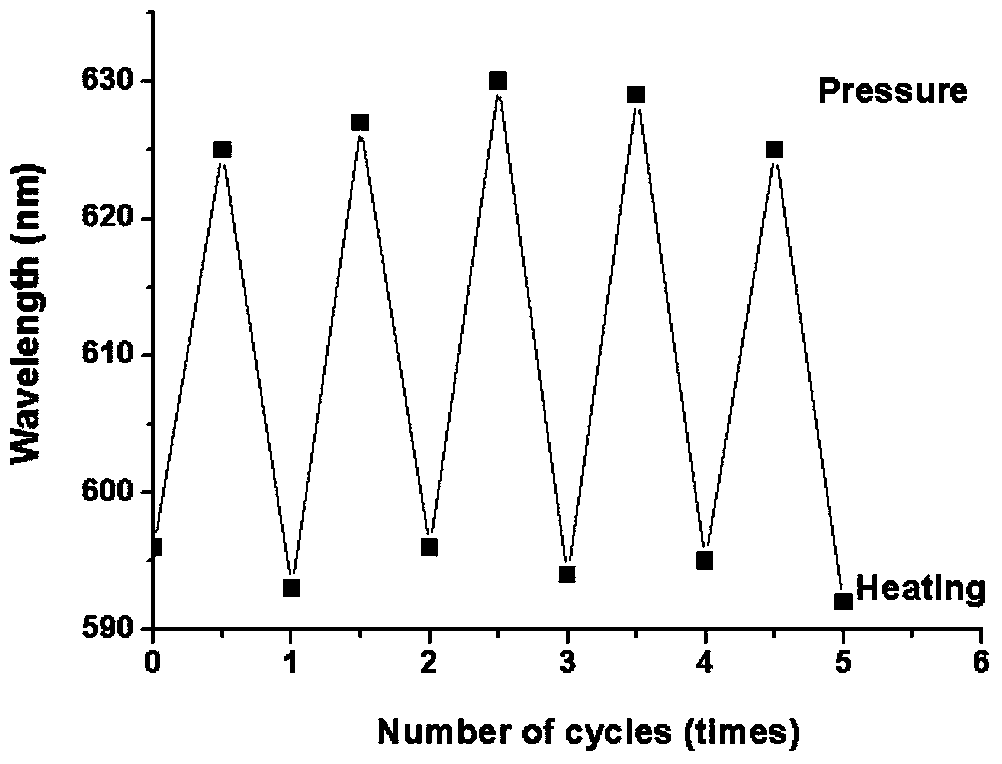
Method for preparing multiple-stimulation respond material containing thienylmaleimide by virtue of dehydration reaction process
A multi-stimuli-responsive, maleimide-based technology, applied in color-changing fluorescent materials, chemical instruments and methods, organic chemistry, etc., can solve problems such as lack of sufficient understanding of piezochromic mechanism, and achieve high quantum efficiency of solid-state fluorescence , simple synthesis steps, good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1) Preparation of 3,4-dichloromaleimide:
[0037] 3.88 g of maleimide (40 mmol) was placed in a high-pressure reaction flask, 20 ml of N,N-dimethylformamide was added, followed by 6.39 ml of thionyl chloride (90 mmol), sealed. Hold the bottle mouth of the high-pressure reaction bottle, heat it to 58 ° C, and gradually a pale yellow solid is produced. After a total of 72 hours of reaction, stop heating and return to room temperature, and filter with 100 ml of water and 60 ml of dichloromethane successively to obtain 3. ,4-dichloromaleimide;
[0038] 2) Preparation of 3,4-dichloromaleic anhydride:
[0039] 0.83 g of 3,4-dichloromaleimide (5 mmol) and 100 ml of 10% NaOH aqueous solution were added to the round-bottomed flask, and the reaction was refluxed for 3 hours. L of hydrochloric acid solution, stop adding the hydrochloric acid solution dropwise after the solid is completely precipitated, stand for 1 hour, filter with suction, and dry to obtain 3,4-dichloromaleic a...
Embodiment 2
[0047] 1) Preparation of 3,4-dibromomaleimide:
[0048] Place 3.88 g of maleimide (40 mmol) in a high pressure reaction flask, add 25 ml of N,N-dimethylformamide, followed by 4.77 ml of bromine (93 mmol), seal the high pressure The bottle mouth of the reaction flask was heated to 58 °C, and a pale yellow solid was gradually produced. After a total of 48 hours of reaction, the heating was stopped and returned to room temperature, and 125 ml of water and 75 ml of dichloromethane were successively used for suction and filtration to obtain 3, 4-dibromomaleimide;
[0049] 2) Preparation of 3,4-dibromomaleic anhydride:
[0050] 1.27 g of 3,4-dibromomaleimide (5 mmol) and 150 ml of 10% NaOH aqueous solution were added to a round-bottomed flask, and the reaction was refluxed for 3 hours. After cooling, 2 mol / L of hydrochloric acid solution, stop adding the hydrochloric acid solution dropwise after the solid is completely precipitated, stand for 1 hour, filter with suction, and dry ...
Embodiment 3
[0056] 1) Preparation of 3,4-dichloromaleimide:
[0057] 3.88 g of maleimide (40 mmol) was placed in a high pressure reaction flask, 30 ml of N,N-dimethylformamide was added, followed by 7.26 ml of thionyl chloride (100 mmol), sealed. Hold the bottle mouth of the high-pressure reaction flask, heat it to 58 ° C, and gradually a pale yellow solid is produced. After a total of 72 hours of reaction, stop heating and return to room temperature, add 150 ml of water and 90 ml of dichloromethane for suction filtration to obtain 3, 4-Dichloromaleimide;
[0058] 2) Preparation of 3,4-dichloromaleic anhydride:
[0059] 0.83 g of 3,4-dichloromaleimide (5 mmol) and 200 ml of 10% NaOH aqueous solution were added to a round-bottomed flask, and the reaction was refluxed for 3 hours. L of hydrochloric acid solution, stop adding the hydrochloric acid solution dropwise after the solid is completely precipitated, stand for 1 hour, filter with suction, and dry to obtain 3,4-dichloromaleic anhydr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com