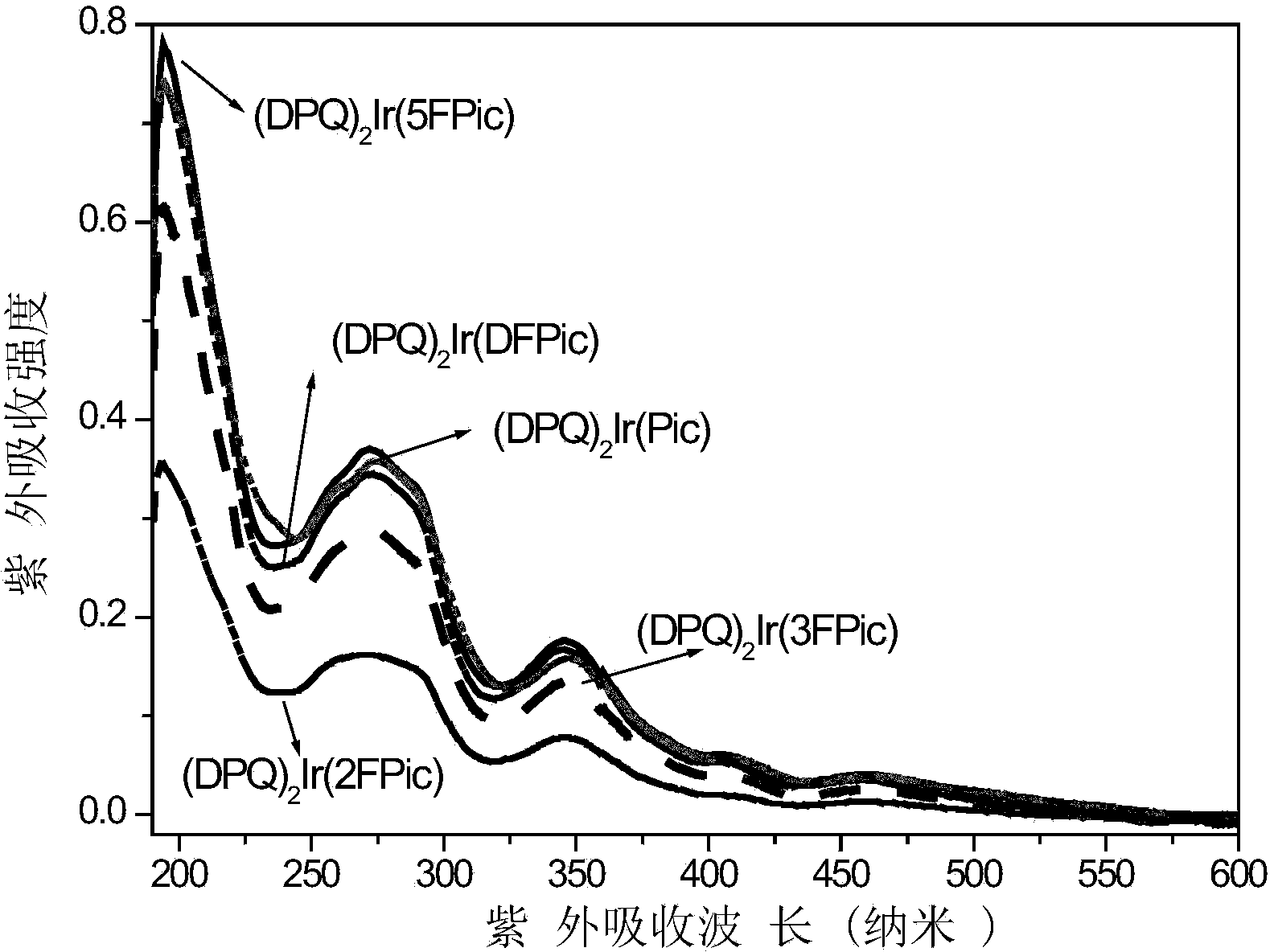
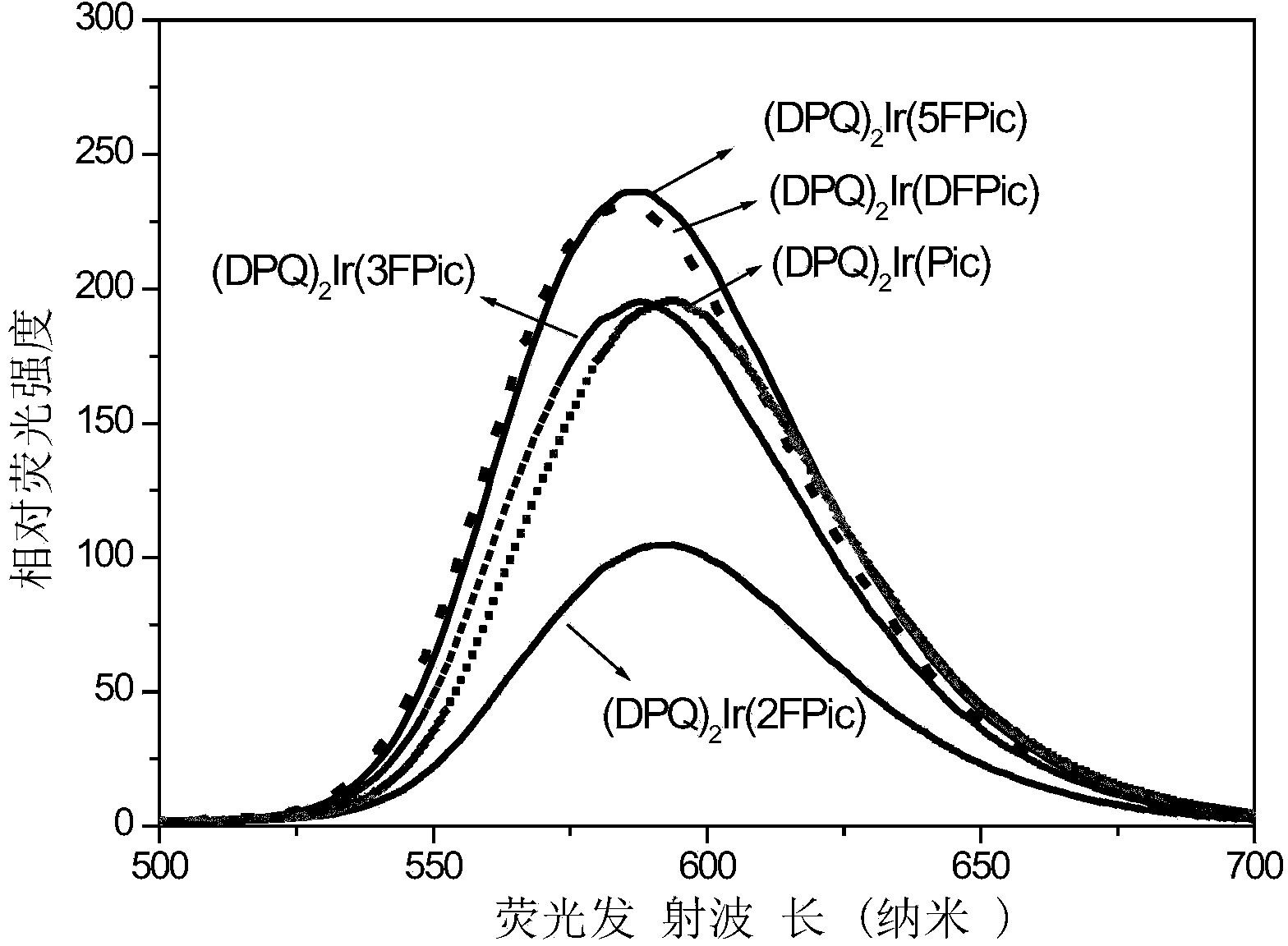
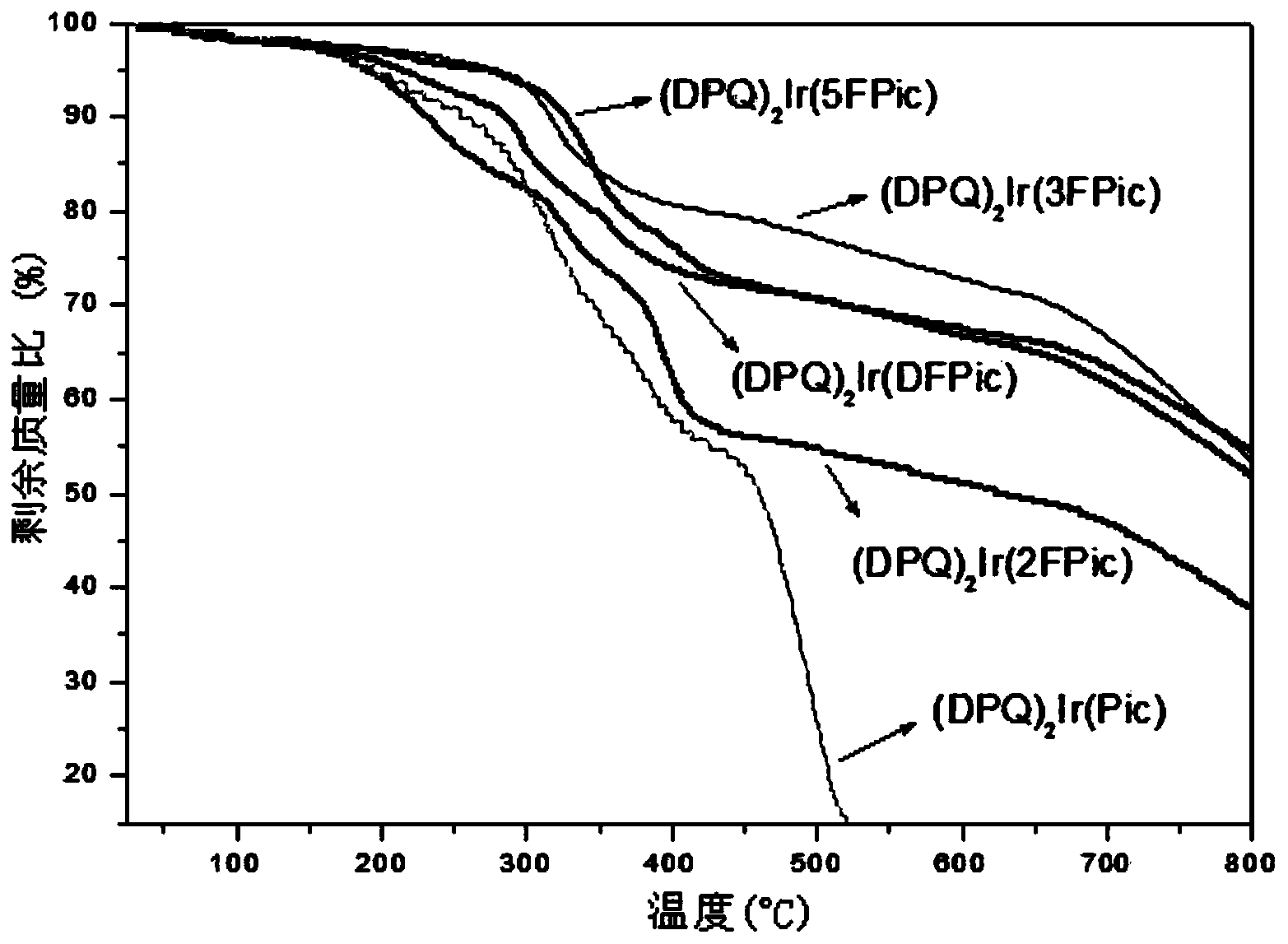
Iridium complex phosphorescent material taking fluorinated fluoropyridine carboxylic acid as auxiliary ligand and preparation method thereof
A technology of fluoropyridine carboxylic acid and iridium complexes, which is applied in the field of iridium complex phosphorescent materials and its preparation, which can solve the problems of increasing the difficulty of synthesis conditions, reducing the luminescence of the complexes, and reducing the photon quantum efficiency, and achieving high internal and external quantum yields , Improve luminous performance, easy separation and purification effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Weigh 1.995g (7.10mmol) 2,4-diphenylquinoline and 1.0g (2.84mmol) IrCl XH 2 O In a two-necked round bottom flask, inject 3mL (165mmol) deionized water and 15mL (465mmoL) ethylene glycol ether, N 2 Under protection, heat up to 110°C for 24 hours, cool to room temperature, filter, wash the solid with deionized water and ethanol, and dry the dichloro bridge compound of iridium (DPQ) 2 Ir.
[0039] Weigh 0.1g (0.127mmol) iridium dichloro bridge compound and dissolve in 20mL dichloromethane, add 0.09g (0.635mmol) 5-fluoro-2-pyridinecarboxylic acid, 12mL ethanol and 2mL triethylamine, under nitrogen protection, Stir at room temperature for 12 h, evaporate the solvent under reduced pressure, and purify by column chromatography on silica gel with dichloromethane / ethyl acetate (6 / 1, volume ratio) to obtain the iridium complex (DPQ) 2 Ir(5FPic). 1 H NMR (CDCl 3 ,400MHz)δ:8.75(d,J=8.0Hz,1H),7.96(s,1H),7.92(s,1H),7.86(d,J=8.0Hz,1H),7.78(t,J=5.2 Hz,2H),7.68(t,J=6.4Hz,3H),7.42-7...
Embodiment 2
[0042] Weigh 1.995g (7.10mmol) 2,4-diphenylquinoline and 1.0g (2.84mmol) IrCl XH 2 O In a two-necked round bottom flask, inject 3mL (165mmol) deionized water and 15mL (465mmoL) ethylene glycol ether, N 2 Under protection, heat up to 110°C for 24 hours, cool to room temperature, filter, wash the solid with deionized water and ethanol, and dry the dichloro bridge compound of iridium (DPQ) 2 Ir.
[0043] Weigh 0.1g (0.127mmol) iridium dichloro bridge compound and dissolve it in 20mL dichloromethane, add 0.09g (0.635mmol) 2-fluoro-6-pyridinecarboxylic acid, 12mL ethanol and 2mL triethylamine, under nitrogen protection, Stir at room temperature for 12 h, evaporate the solvent under reduced pressure, and purify by column chromatography on silica gel with dichloromethane / ethyl acetate (6 / 1, volume ratio) to obtain the iridium complex (DPQ) 2 Ir(2FPic). 1 H NMR (CDCl 3 ,400MHz)δ:8.92(d,J=8.8Hz,1H),8.05(s,1H),7.99(s,1H),7.89(d,J=7.6Hz,1H),7.79(d,J=7.6 Hz,4H),7.69-7.76(m,1H),7.55-7...
Embodiment 3
[0046] Weigh 1.995g (7.10mmol) 2,4-diphenylquinoline and 1.0g (2.84mmol) IrCl XH 2 O In a two-necked round bottom flask, inject 3mL (165mmol) deionized water and 15mL (465mmoL) ethylene glycol ether, N 2 Under protection, heat up to 110°C for 24 hours, cool to room temperature, filter, wash the solid with deionized water and ethanol, and dry the dichloro bridge compound of iridium (DPQ) 2 Ir.
[0047] Weigh 0.1g (0.127mmol) iridium dichloro bridge compound and dissolve it in 20mL dichloromethane, add 0.09g (0.635mmol) 3-fluoro-2-pyridinecarboxylic acid, 12mL ethanol and 2mL triethylamine, under nitrogen protection, Stir at room temperature for 12 h, evaporate the solvent under reduced pressure, and purify by column chromatography on silica gel with dichloromethane / ethyl acetate (6 / 1, volume ratio) to obtain the iridium complex (DPQ) 2 Ir(3FPic). 1 H NMR (CDCl3 ,400MHz)δ:8.89(d,J=8.8Hz,1H),8.04(s,1H),8.01(s,1H),7.93(d,J=7.6Hz,1H),7.90(d,J=4.8 Hz,1H),7.78(t,J=7.2Hz,4H),7.54-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com