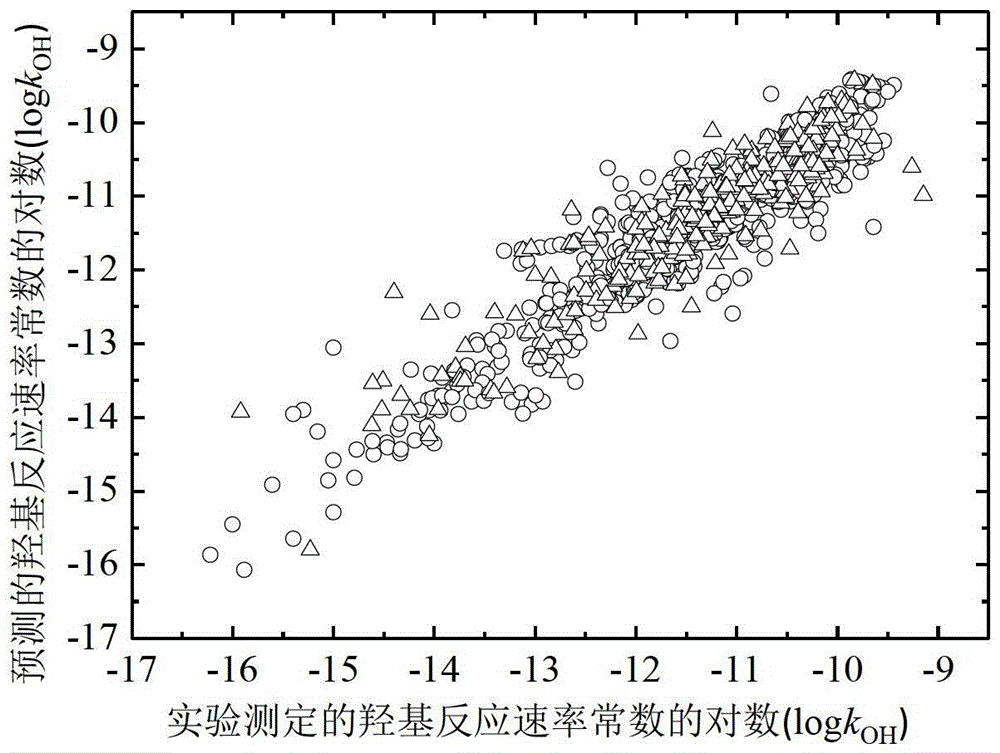
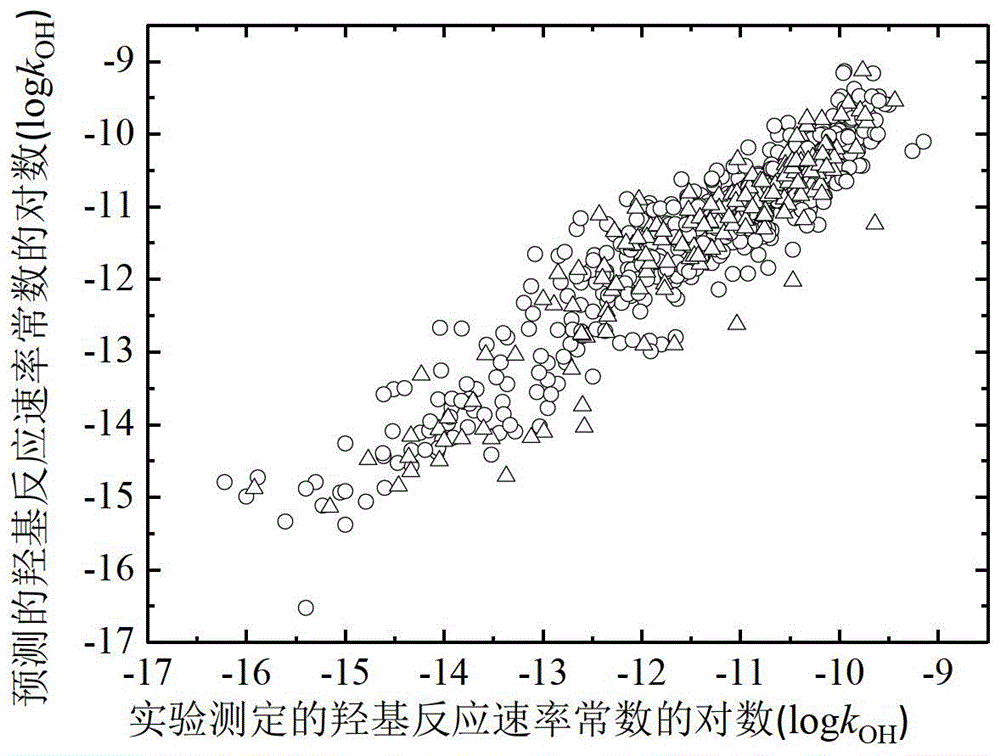
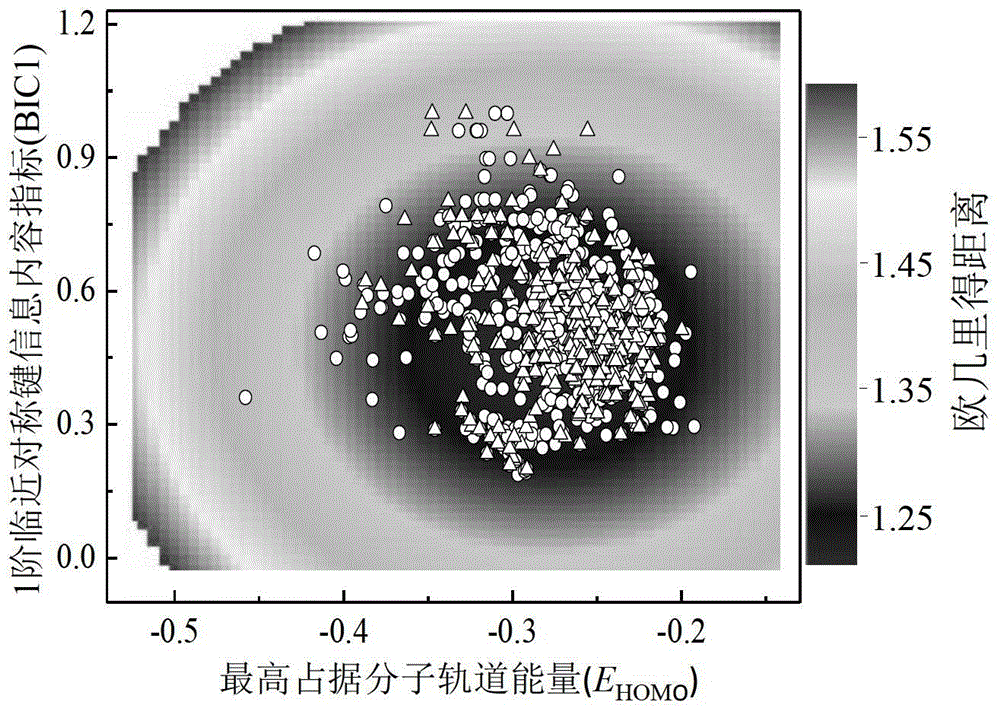
A Method for Predicting the Rate Constant of the Reaction of Organic Compounds with Hydroxyl in the Atmosphere Using Quantitative Structure-Activity Relationship Model
A technique for quantifying structure-activity relationships and reaction rate constants, applied in electrical digital data processing, special data processing applications, instruments, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Given a compound butyl methacrylate, to predict its logk at 287K, 294K, 298K, 303K and 313K OH value. First, according to the structural information of butyl methacrylate, after using Gaussian09 software to optimize its structure, E can be calculated. HOMO The value is -0.2752, X% is calculated by Draogon6.0 software, the values of Mor29u, NdsCH, GATS1e, X3A, SdsCH, BIC1, RDF015m, SpMin8_Bh(p), nR=Cp, NssssC and F02[F-Br] are respectively 0, -0.84, 0, 0.708, 0.267, 0, 0.565, 5.99, 0, 1, 0 and 0. Then according to formula (4), the Euclidean distance of the feature vector is 0.5316 (OH The predicted values at T=287K, 294K, 298K, 303K and 313K are -10.48, -10.47, -10.46, -10.45 and -10.43. Instead of logk at T=287K, 294K, 298K, 303K and 313K OH The experimental data -10.12, -10.16, -10.18, -10.20 and -10.25 are fitted, and the correlation coefficient R between the two is obtained 2 =0.986, the predicted value is in good agreement with the experimental data.
Embodiment 2
[0041]Given a compound 2,3-dimethylbutane with a large amount of experimental data, it is predicted that it is logk at 1082K, 1107K, 1144K, 1156K, 1161K, 1183K, 1206K, 1231K, 1247K, 1249K and 1292K OH value and compared with the experimental value. According to the structural information of 2,3-dimethylbutane, after using Gaussian09 software to optimize its structure, the E HOMO The value is -0.30974, calculated by Draogon6.0 software X%, Mor29u, NdsCH, GATS1e, X3A, SdsCH, BIC1, RDF015m, SpMin8_Bh(p), nR=Cp, NssssC and F02[F-Br] are respectively 0, -0.868, 0, 1.667, 0.333, 0, 0.272, 4.225, 0, 0, 0, 0. Then according to the formula (4), the Euclidean distance of the feature vector is 0.4967 (OH At 298K, 843K, 869K, 872K, 887K, 893K, 937K, 938K, 951K, 977K, 1009K, 1038K, 1039K, 1043K, 1082K, 1107K, 1144K, 1156K, 1161K, 1183K, 1206K, 1231K, 2949K logk at temperature OH Predicted values are -11.11, -10.74, -10.74, -10.74, -10.73, -10.73, -10.72, -10.72, -10.72, -10.72, -10.7...
Embodiment 3
[0043] Given a compound p-xylene, predict its logk at T=298K OH And compared with the experimental value. According to the structural information of 2,3-dimethylbutane, after using Gaussian09 software to optimize its structure, the E HOMO The value is -0.23683, AMW, NdsCH, Mor14i, nR=Cp, nP, nRCHO, X%, SpMaxA_AEA(dm), C-020, nCbH, CATS2D_03_D calculated by Draogon6.0 software are 5.899, 0, 0.533, 0 respectively , 0, 0, 0, 0.34, 0, 4, 0. Then according to the formula (4), the Euclidean distance of the feature vector is 0.3876 (OH The predicted value of -10.78,. Instead of logk at T=298K OH Experimental data -10.86 contrast, the difference is 0.08. The predicted value is very close to the experimental value, indicating that its predictability is good.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com