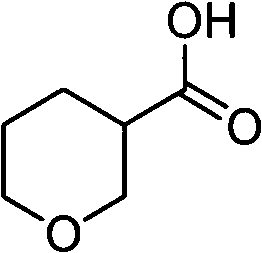
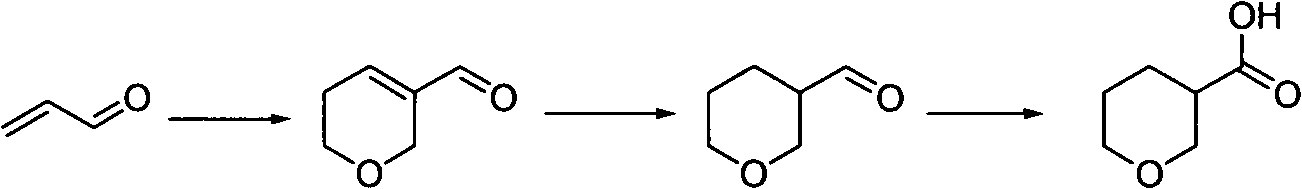
Tetrahydropyrane-3-formic acid preparation method
A technology of tetrahydropyran and formic acid, which is applied in the direction of organic chemistry, can solve the problems of expensive silver nitrate, high cost, and unsuitability for industrial production, and achieve the effects of low production cost, mild chemical reaction conditions, and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020] The present invention will be described in detail below through specific embodiments, but the present invention is not limited to these examples.
[0021] Add 5L of water, 840mL of concentrated hydrochloric acid and 600mL of toluene into a 20L reaction kettle, add 1Kg of acrolein, heat to an internal temperature of 65-75 degrees under nitrogen protection, and react for about 2 hours. The reaction solution was cooled, filtered, and separated into layers. The toluene layer was neutralized with aqueous sodium bicarbonate until there were no bubbles, dried, and the solvent was removed by rotary evaporation to obtain a crude product. The aqueous layer was extracted with dichloromethane, 2.5 L / time, four times, the organic layers were combined, neutralized with aqueous sodium bicarbonate solution, dried, and the solvent was removed by rotary evaporation to obtain a crude product. The two crude products were combined and distilled under reduced pressure to obtain 5,6-dihydro-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com