Hematoxylin staining method
A technology of hematoxylin and hematin, applied in the field of biological sample staining
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
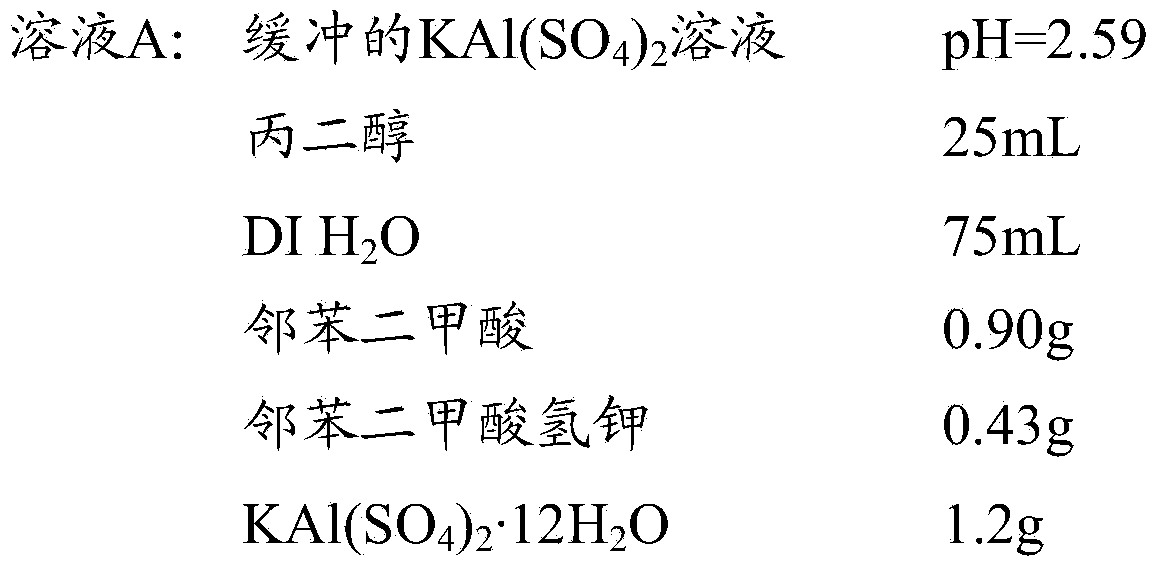
[0029] Embodiment 1: the preparation of solution
[0030] Prepare the following solutions:
[0031]
[0032]
[0033] For solutions A and B, the propylene glycol was heated on a hot plate at 60 °C. The phthalic acid is added, and the composition is heated until the solids dissolve. Turn off the heat and add potassium hydrogen phthalate in DI H to the propylene glycol solution with stirring. 2 solution in O. Add solid potassium aluminum sulfate to solution A. Solution A was then stirred overnight and then filtered using 25 micron fluted filter paper. Hematoxylin was added to Solution B, and the composition was stirred for about 15 minutes. Solid sodium iodate was then added and the solution was stirred overnight, then filtered using 25 micron grooved filter paper.
Embodiment 2
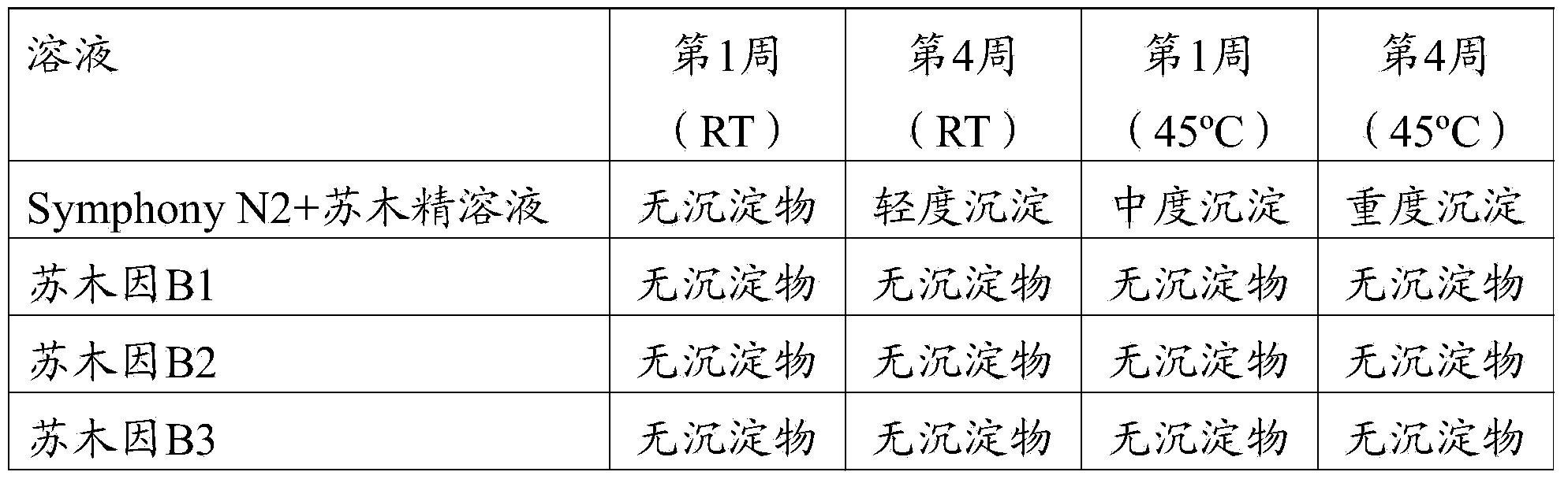
[0034] Example 2: Staining of Biological Samples
[0035] Material: KAl(SO 4 ) 2 Buffered solution (double strength; 2x solution A); hematoxylin buffered solution (double strength; 2x solution B), 50 / 50 propylene glycol / H with 1% Merpol wash 2O; 50 / 500.1 Tris blue dye (blueing); 95% propylene glycol; transfer fluid (Transfer fluid); 3X3MTB slides.
[0036] Use the following protocol on thick film (open) cassettes. Slides were manually deparaffinized, lightly blotted and placed in boxes. Dispense 400 μL of 2X Solution A onto the tissue, followed immediately by dispensing 400 μL of 2X Solution B on the tissue. Slides were then incubated for 3 minutes. Slides were then rinsed twice for 10 s with 800 μL of 1% aqueous Merpol. Slides were then treated with 800 µL of 95% propylene glycol for 10 s. Slides were then rinsed 3 times for 10 seconds each with 800 [mu]L of transfer fluid. Coverslips were then applied on a Sakkura TissueTek instrument.
[0037] Use this protocol on ...
Embodiment 3
[0038] Example 3: Preparation of two-part staining solution
[0039] Solution A: 0.75M phthalate-buffered KAl(SO 4 ) 2 . To 250ml of propylene glycol was added 8.97g of phthalic acid and the mixture was stirred at 60°C until the solids dissolved. Add potassium hydrogen phthalate solution (4.3 g in 750 mL DI H 2 O), and remove the solution from the heat source. Then add 12.0g KAl (SO 4 ) 2 12H 2 O, the mixture was stirred until it was homogeneous. The solution is then filtered. The pH is 2.59.
[0040] Solution B: 0.75M Phthalate Buffered Hematine. To 250 mL of propylene glycol was added 8.97 g of phthalic acid, and the mixture was stirred at 60°C until the solids dissolved. Add potassium hydrogen phthalate solution (4.3 g in 750 mL DI H 2 O), and remove the solution from the heat source. Add 12.0 g of hematoxylin with stirring. After 15-20 minutes, 1.2 g of sodium iodate was added and the mixture was stirred overnight. The solution is then filtered. The pH is 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com