Preparation method of immobilized alkaline ionic liquid catalyst
A liquid catalyst, basic ion technology, applied in physical/chemical process catalysts, chemical instruments and methods, organic compound/hydride/coordination complex catalysts, etc., can solve the problem of high cost, low catalyst activity, difficult recovery, etc. problem, to achieve the effect of reducing preparation cost, high catalyst activity, and no pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Add toluene, pure silicon molecular sieve MCM-41 and chloroacetyl chloride in sequence in a round bottom flask, wherein the mass ratio of pure silicon molecular sieve MCM-41 to toluene is 1:30, pure silicon molecular sieve MCM-41 and chloroacetyl chloride The mass ratio is 8:1. Magnetically stirred and reacted at -10°C for 6 hours to obtain pure silicon molecular sieves with esterified hydroxyl groups on the surface.
[0028] (2) After filtering, washing and drying the reaction system obtained in step (1), put the obtained solid into a dry round bottom flask, and then add toluene and 1-methylimidazole in sequence, wherein the mass of toluene and solid The ratio is 10:1, the mass ratio of 1-methylimidazole to solid is 0.2:1, heated to 60°C with magnetic stirring for 6 hours, and the immobilized ionic liquid with pure silicon molecular sieve as carrier can be obtained.
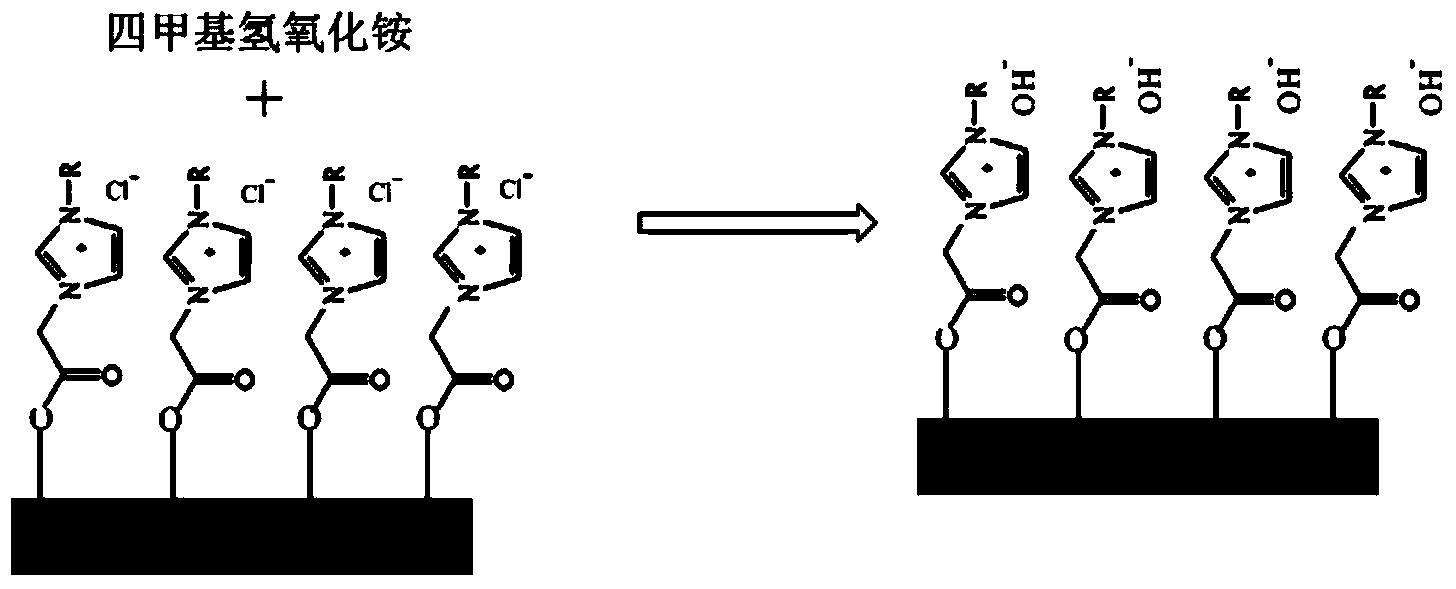
[0029] (3) Add the immobilized ionic liquid obtained in step (2) into a round-bottomed flask, and...
Embodiment 2
[0031] (1) Add toluene, pure silicon molecular sieve MCM-48 and chloroacetyl chloride in sequence in a round bottom flask, wherein the mass ratio of pure silicon molecular sieve MCM-48 to toluene is 1:30, pure silicon molecular sieve MCM-48 and chloroacetyl chloride The mass ratio is 8:1. The reaction was carried out under magnetic stirring at 30° C. for 6 hours to obtain a pure silicon molecular sieve with esterified hydroxyl groups on the surface.
[0032] (2) After filtering, washing and drying the reaction system obtained in step (1), add the obtained solid into a round bottom flask, and then add toluene and 1-methylimidazole in sequence, wherein the mass ratio of toluene to solid is 10 : 1, The mass ratio of 1-ethylimidazole to solid is 0.5:1, heated to 100° C. for 6 hours with magnetic stirring, and the immobilized ionic liquid with pure silicon molecular sieve as carrier can be obtained.
[0033] (3) Add the immobilized ionic liquid obtained in step (2) into a round bo...
Embodiment 3
[0035] (1) Add toluene, pure silicon molecular sieve SBA-15 and chloroacetyl chloride in sequence in a round bottom flask, wherein the mass ratio of pure silicon molecular sieve SBA-15 to toluene is 1:30, pure silicon molecular sieve SBA-15 and chloroacetyl chloride The mass ratio is 8:1. The reaction was carried out under magnetic stirring at 10° C. for 6 hours to obtain pure silicon molecular sieves with esterified hydroxyl groups on the surface.
[0036] (2) After filtering, washing and drying the reaction system obtained in step (1), add the obtained solid into a round bottom flask, and then add toluene and 1-propylimidazole in sequence, wherein the mass ratio of toluene to solid is 10 : 1, The mass ratio of 1-propylimidazole to solid is 0.3:1, and heated to 100° C. for 6 hours with magnetic stirring to obtain an immobilized ionic liquid with pure silicon molecular sieve as the carrier.
[0037] (3) Add the immobilized ionic liquid obtained in step (2) into a round bottom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com