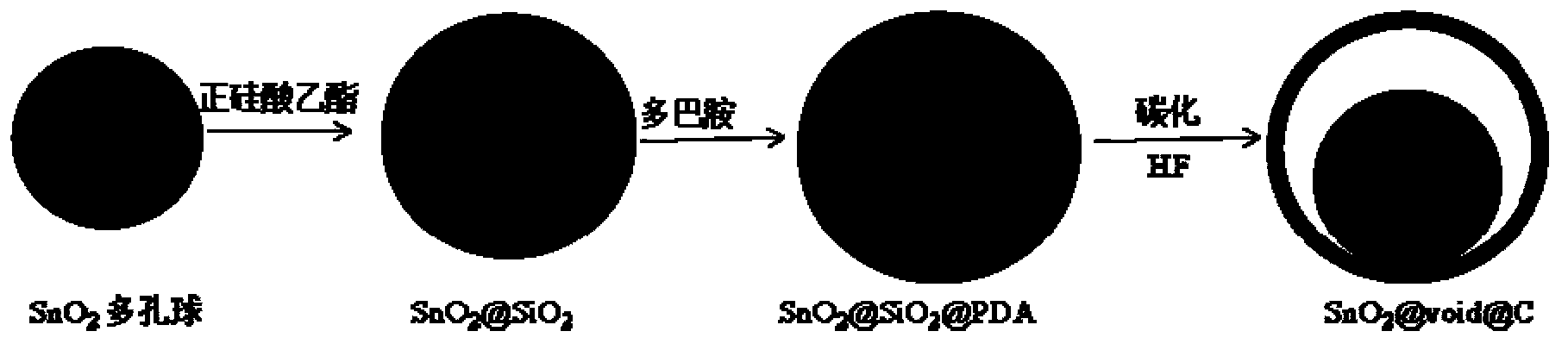
Yolk-shell structure tin dioxide-nitrogen-doped carbon material and preparation method thereof
A technology of tin dioxide and nitrogen-doped carbon, which is applied to structural parts, nanotechnology for materials and surface science, electrical components, etc., can solve problems such as easy agglomeration and poor structural stability, and achieve improved conductivity and operation Convenience, the effect of controlling the size of the gap
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Take 80 mL of a mixed solvent with a volume ratio of ethanol and water of 3:5, and prepare K 2 SnO 3 ·3H 2 The solution with O concentration of 15 mmol / L and urea concentration of 0.45 mol / L was stirred evenly and then transferred to a 100 mL polytetrafluoroethylene-lined autoclave and placed in an oven for 140 o C was heated at a constant temperature for 2 hours, and the autoclave was taken out and cooled to room temperature naturally to obtain a white precipitate, which was washed with absolute ethanol and centrifuged for 3 times to remove the metal ion on the precipitate, at 60 o C dried for 12 hours to obtain porous SnO 2 ball.
[0024] 100 mg of the above porous SnO 2 The balls were dispersed in a mixed solvent of 160 mL of ethanol and 40 mL of water, ultrasonically mixed, and then magnetically stirred. During the stirring process, 3 mL of ammonia water with a mass fraction of 25% was slowly added, followed by 1.2 g of 0.92 g / mL of tetraethyl orthosilicate TE...
Embodiment 2
[0029] Take 50 mL of a mixed solvent with a volume ratio of ethanol and water of 3:5, and prepare K 2 SnO 3 ·3H 2 The solution with O concentration of 16 mmol / L and urea concentration of 0.5 mol / L was stirred evenly and transferred to a 100 mL polytetrafluoroethylene-lined autoclave, and placed in an oven for 145 o C was heated at a constant temperature for 2.5 hours, and the autoclave was taken out and cooled to room temperature naturally to obtain a white precipitate, which was washed with absolute ethanol and centrifuged 4 times to remove the metal ion on the precipitate, at 70 o C dried for 10 hours to obtain porous SnO 2 ball.
[0030] 80 mg of the above porous SnO 2 The spheres were dispersed in a mixed solvent of 160 mL ethanol and 40 mL water, ultrasonically mixed to make them uniform, and then magnetically stirred. During the stirring process, 4 mL of ammonia water with a mass fraction of 26% was slowly added, followed by 2.4 g of 0.93 g / mL of tetraethyl orthosi...
Embodiment 3
[0033] Take 50 mL of a mixed solvent with a volume ratio of ethanol and water of 3:5, and prepare K 2 SnO 3 ·3H 2 The solution with O concentration of 17 mmol / L and urea concentration of 0.55 mol / L was stirred evenly and transferred to a 100 mL polytetrafluoroethylene-lined autoclave, and placed in an oven for 150 o C was heated at a constant temperature for 3 hours, and the autoclave was taken out and cooled to room temperature naturally to obtain a white precipitate, which was washed with absolute ethanol and centrifuged 5 times to remove the metal ion on the precipitate, at 80 o C dried for 8 hours to obtain porous SnO 2 ball.
[0034] 120 mg of the above porous SnO 2The spheres were dispersed in a mixed solvent of 160 mL ethanol and 40 mL water, ultrasonically mixed evenly, and then magnetically stirred. During the stirring process, 2 mL of ammonia water with a mass fraction of 27% was slowly added, followed by 3.6 g of 0.94 g / mL of tetraethyl orthosilicate TEOS, sti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com