Mammal cell expression system
A mammalian cell technology, applied in the field of efficient and stable mammalian cell expression system, can solve the problems of inconvenient screening work and achieve the effects of reducing the probability of false positive clones, reducing the size of the vector, and reducing the burden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: Construction of mammalian cell expression vector
[0044] Methods: The traditional expression vector pA was constructed by molecular biology techniques, such as restriction enzyme digestion, DNA ligation, Escherichia coli transformation and clone screening, according to the standard methods of "Molecular Cloning Experiment Guide" (third edition, translated by Huang Peitang, Science Press) And the high expression vector pB of the present invention. The specific operation method is as follows: Using the pcDNA3.1 vector of Invitrogen Company as a template, artificially synthesize the fusion sequence (1.8 kb) of EF1-α promoter, DHFR, HSV TK terminator and other regulatory sequences, and add SpeI and NaeI at the end respectively. Restriction sites. The synthetic fusion sequence was connected to pcDNA3.1 double-digested by SpeI and NaeI using T4DNA ligase, transformed into Escherichia coli, and carried out single clone screening to obtain the traditional vector...
Embodiment 2
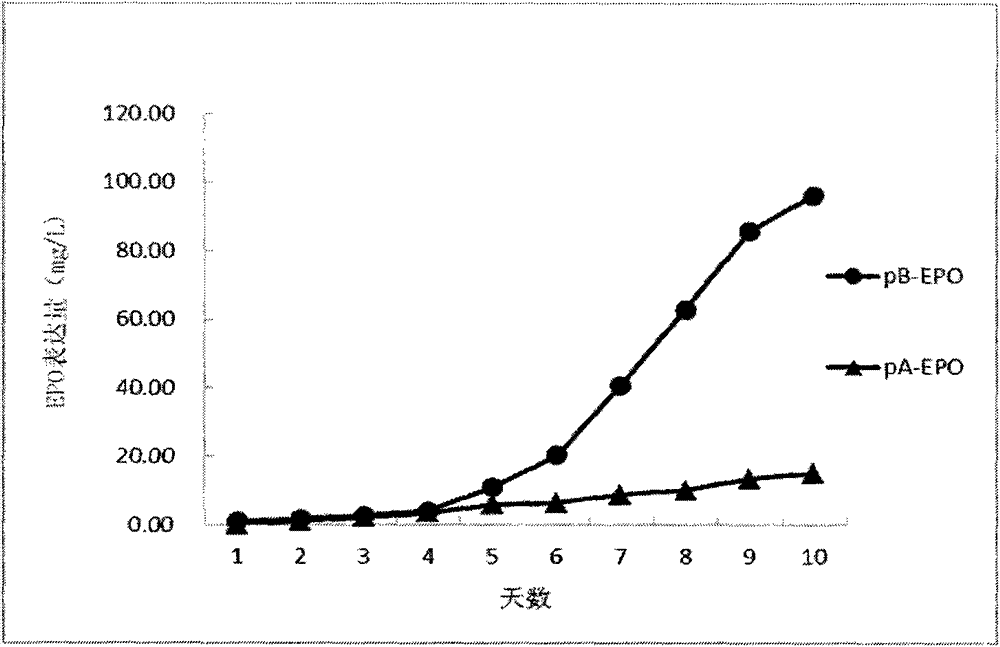
[0069] Embodiment 2: the comparison of the expression amount of EPO protein in the traditional expression system and the expression system of the present invention
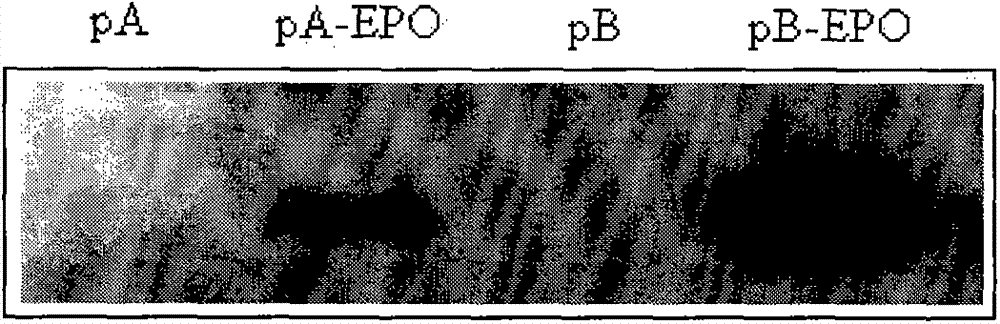
[0070] 2.1. Construction of an expression vector containing the target gene: artificially synthesize the coding sequence of EPO according to the existing sequence information of NCBI, and clone it into the expression vector constructed in Example 1 by introducing corresponding restriction sites (NheI and HindIII) Among pA and pB, the expression vectors pA-EPO and pB-EPO containing the target gene were obtained.
[0071] 2.2. DNA transfection into CHO-dhfr - Suspension cell lines: Transfect the above-mentioned expression vectors pA-EPO and pB-EPO containing the target gene, and expression vector control pA and pB into DHFR enzyme-deficient CHO cells (CHO-dhfr - )middle. CHO-dhfr - The cells were cultured in suspension, and the cell density was 1.5×10 6 / m], the transfection volume was 30ml. The transfection me...
Embodiment 3
[0084] Embodiment 3: the comparison of the expression amount of EPO protein in the expression system containing different promoter vectors
[0085] 3.1. Experimental method: the experimental method of constructing the expression vector containing the target gene, DNA transfection into CHO-dhfr - The method for screening suspension cells and stable cells is the same as that in Example 2. In order to construct expression vectors containing different promoters before the target gene, the CMV promoters in the expression vector pB containing the EPO target gene in Example 2 were replaced with artificially synthesized MPSV promoters and RSV promoters respectively, and then the above-mentioned constructed The expression vector pB-EPO containing different promoters and EPO were transfected into DHFR enzyme-deficient CHO cells (CHO-dhfr - ), a stable expression cell line was obtained after initial screening with Zeocin selection medium and MTX gradient pressurization screening.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com