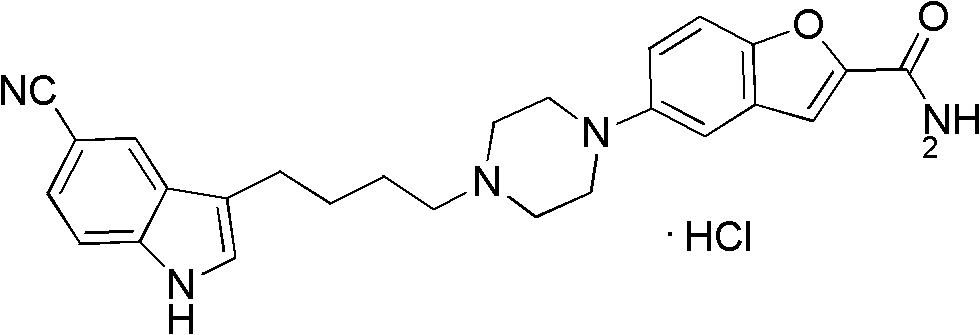
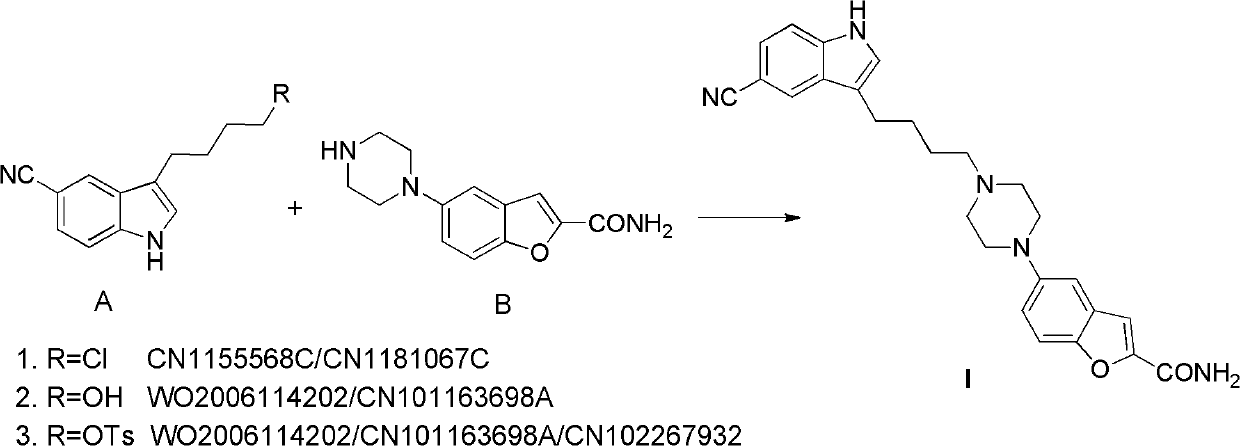
Preparation method of antidepressant drug-vilazodone
A preparation process and catalyst technology, applied in the direction of organic chemistry, etc., can solve the problems of low yield, difficult industrial scale-up production, unfavorable product purification, etc., and achieve the effect of easy availability of reaction raw materials and convenient post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
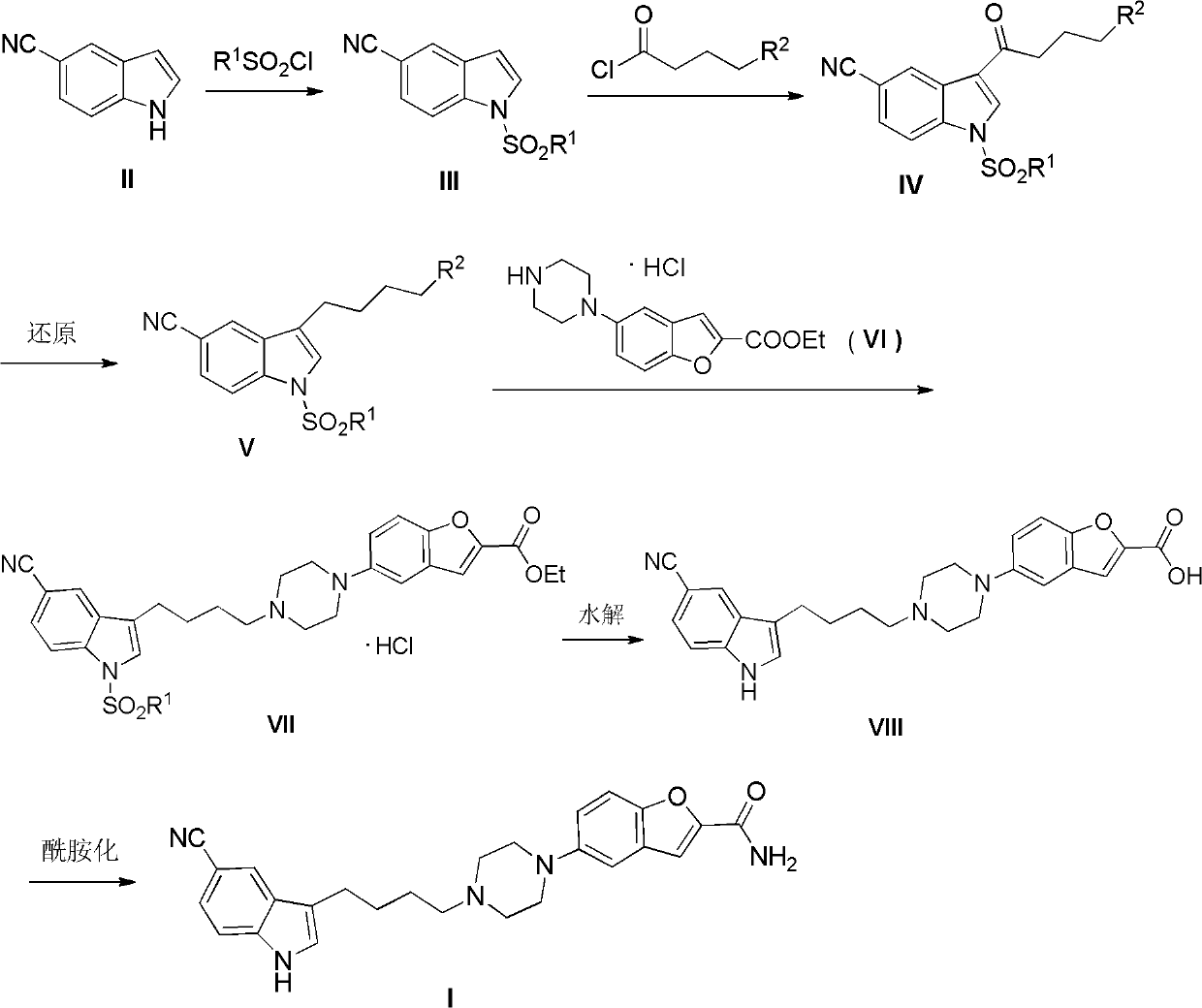
[0028] Synthesis of 1-p-toluenesulfonyl-5-cyanindole (III)
[0029] In a 1L three-necked flask, 5-cyanoindole (10.0g, 0.07mol), toluene (300ml), 30% NaOH (300ml) solution and tetrabutylammonium bromide (2.3g, 7mmol) were mixed at room temperature p-Toluenesulfonyl chloride (14.8 g, 0.074 mol) was added under conditions. Stir for 1 hour to stop the reaction. Extract with dichloromethane (3×125ml), combine the organic layers, wash with saturated brine, and dry over anhydrous sodium sulfate overnight. Suction filtration, the filtrate was spin-dried to obtain 20.4g of white solid, yield 98.01%, m.p.116~118℃.(Ref.m.p.116~118℃.Bioorg.Med.Chem.Lett.2010,20(12),3534-3536 )
Embodiment 2
[0031] Synthesis of 1-p-toluenesulfonyl-3-(4-chlorobutyryl)-5-cyano-1H-indole (IV)
[0032] In a 1L three-necked flask, mix aluminum trichloride (18.7g, 0.14mol), dichloromethane (600ml), 4-chlorobutyryl chloride (12.5g, 0.088mol), add 1-p-toluenesulfonyl-5 - Cyanoindole (20 g). After continuing to stir and react for 8 h, the reaction solution was poured into ice water (600 ml), extracted with ethyl acetate (3×500 ml), the organic layers were combined, washed with saturated brine, and dried overnight over anhydrous sodium sulfate. After suction filtration, the filtrate was spin-dried to obtain 24.3 g of white solid, with a yield of 89.80%, m.p.154-156°C.
[0033] 1H-NMR (300MHz, CDCl 3), δ (ppm): 8.71 (1H, d, J = 1.4Hz, 4-ArH), 8.36 (1H, s, 2-ArH), 8.03 (1H, d, J = 8.7Hz, 7-ArH), 7.86 (2H, d, J = 8.3Hz, 1'-ArH), 7.62 (1H, dd, J = 8.7Hz, J = 1.4Hz, 6-ArH), 7.34 (2H, d, J = 8.3Hz, 2 '-ArH), 3.69 (2H, t, J=6.3Hz, -CH2Cl), 3.13 (2H, t, J=6.3Hz, -COCH2-), 2.26 (2H, qt, J=6.3Hz,...
Embodiment 3
[0035] Synthesis of 1-p-toluenesulfonyl-3-(4-chlorobutyl)-5-cyano-1H-indole (V)
[0036] In a 1L three-necked flask, mix sodium borohydride (34.0g, 0.9mol), dichloromethane (400ml), trifluoroacetic acid (400ml), stir for 0.5 hours and add 1-p-toluenesulfonyl-3-(4 -Chlorobutyryl)-5-cyano-1H-indole (20.0g, 50mmol), the reaction was continued for 8h and stopped. The reaction solution was extracted with ethyl acetate (3×750ml), and the organic layers were combined, washed with saturated sodium carbonate and brine, and dried over anhydrous sodium sulfate overnight. Suction filtration, the filtrate was spin-dried to obtain 18.4g white solid, yield 95.33%, m.p.110~112℃.
[0037] 1H-NMR (300MHz, CDCl 3 ), δ (ppm): 8.06 (1H, d, J = 8.6Hz, 7-ArH), 7.81 (1H, s, 4-ArH), 7.75 (2H, d, J = 8.3Hz, 1'-ArH) , 7.56 (1H, d, J = 8.6Hz, 6-ArH), 7.46 (1H, s, 2-ArH), 7.25 (2H, d, J = 8.3Hz, 2'-ArH), 3.57 (2H, m , -CH2Cl), 2.70 (2H, t, m, Ar-CH 2 ), 2.36 (3H, s, -CH 3 ), 1.84 (4H, m, -CH 2 CH ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com