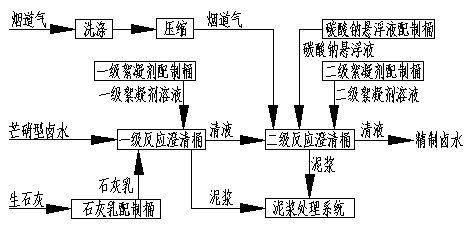
Purifying process of mirabilite type brine by adopting lime-carbon dioxide process
A carbon dioxide and brine purification technology, which is applied in chemical instruments and methods, inorganic chemistry, alkali metal chlorides, etc., can solve the problems of increasing the purification cost of mirabilite-type brine, rising prices of soda ash, etc., and achieve reduced steam consumption and long production cycle , The effect of reducing carbon dioxide emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1 adopts attached figure 1 Intermittent Glauber's salt type brine purification process; Embodiment 2 adopts attached figure 2 Continuous Glauber's salt type brine purification process; Embodiment 3 adopts attached image 3 Intermittent Glauber's salt type brine purification process; Embodiment 4 adopts attached Figure 4 continuous mirabilite type brine purification process.
[0037] Example 1:
[0038] The composition of Glauber's salt brine is: H 2 O: 880g / l; NaCl: 295g / l; Na 2 SO 4 : 23g / l; CaSO 4 : 1.5g / l; MgSO 4 : 0.25g / l; the salt nitrate workshop produces 10t of industrial salt and 0.7t of industrial sodium sulfate as a by-product, so the purification workshop needs to process 35.7m 3Glauber's salt-type brine, first-order reaction, add 34.1kg CaO at a stirring speed of 20-100r / min, continue to stir and react for 5 hours, then add 0.07kg of a first-class flocculant, stop stirring for 2 hours, and measure the Mg in the clear liquid 2+ and pH va...
Embodiment 2
[0040] The composition of Glauber's salt brine is: H 2 O: 881.4g / l; NaCl: 295 g / l; Na 2 SO 4 : 22g / l; CaSO 4 : 1.4g / l; MgSO 4 : 0.22g / l; the salt nitrate workshop produces 10t of industrial salt and 0.7t of industrial sodium sulfate as a by-product, so the purification workshop needs to process 35.7m 3 Glauber's salt-type brine, in the primary reaction, add 33.6 kg of CaO at a continuous stirring speed of 20 to 100 r / min, and ensure that the residence time is more than 5 hours, transfer to the primary clarification equipment, and add 0.07 kg of primary flocculant at the same time, measure the Mg 2+ and PH values are 0.1ppm and 12.5 respectively; the clear liquid from the primary clarification equipment is transferred to the secondary reaction barrel, and the secondary reaction is fed into the flue gas 149Nm at a continuous stirring speed of 20-100r / min 3 , and add 10.1kg of soda ash, and ensure that the residence time is more than 2 hours, transfer to the secondary clar...
Embodiment 3
[0042] The composition of Glauber's salt brine is: H 2 O: 891g / l; NaCl: 290g / l; Na 2 SO 4 : 16.8g / l; CaSO 4 : 2.04g / l; MgSO 4 : 0.25g / l; if the salt nitrate workshop produces 10t of industrial salt, the purification workshop needs to process 36.1m 3 Glauber's salt-type brine, while reducing the production of industrial sodium sulfate in the salt nitrate workshop, using the salt nitrate workshop to return 9.4 m 3 The salt nitrate mother liquor is mixed with it, thereby reducing the output of sodium sulfate in the salt nitrate workshop. Glauber's salt-type brine and salt salt mother liquor are mixed in the first-stage reaction, and 42.8kg CaO is added at a stirring speed of 20-100r / min to continue stirring and reacting for 5 hours. Mg 2+ and pH values are 0.1ppm and 12.5 respectively; transfer the first-stage clear liquid to the second-stage reaction, and the second-stage reaction is passed into the flue gas 219Nm at a stirring speed of 20-100r / min 3 , continue to stir ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com