Application of medicine composition in preparation of medicines for preventing or treating fatty liver
A composition and fatty liver technology, applied in the field of medicine, to achieve the effects of improving animal liver lesions, preventing and treating the occurrence and development of animal liver lesions, and alleviating the degree of animal liver lesions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
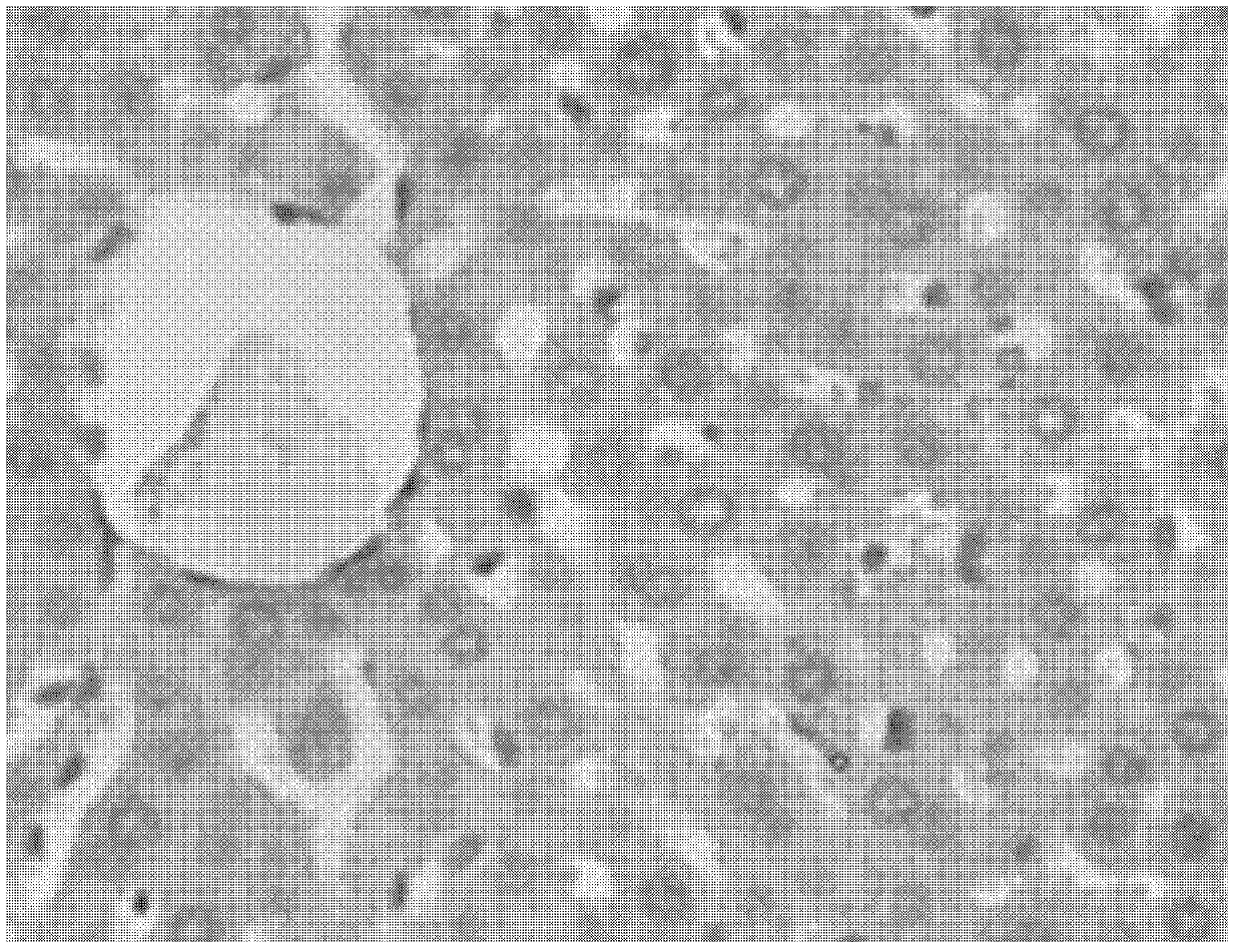
[0046] Embodiment 1. The effect of the pharmaceutical composition on the fatty liver model of acute carbon tetrachloride liver injury in mice
[0047] 1. Experimental method
[0048] Male mice of Kunming species were randomly divided into 6 groups according to body weight, 12 in each group, which were respectively normal group, model group, and positive drug were clinically commonly used ursodeoxycholic acid capsule group, pharmaceutical composition (according to embodiment six Preparation) low, medium and high dose groups (0.5g drug / kg body weight, 1g drug / kg body weight, 2g drug / kg body weight). The test product group was administered by intragastric administration, 1 time / day, the normal group and the model group were given equal-volume distilled water, and the volume of intragastric administration was 0.2ml / 10g body weight, and the administration was continued for 10 days; on the 10th day, except for the blank group, For the rest of the animals, gavage mice with 0.15% car...
Embodiment 2
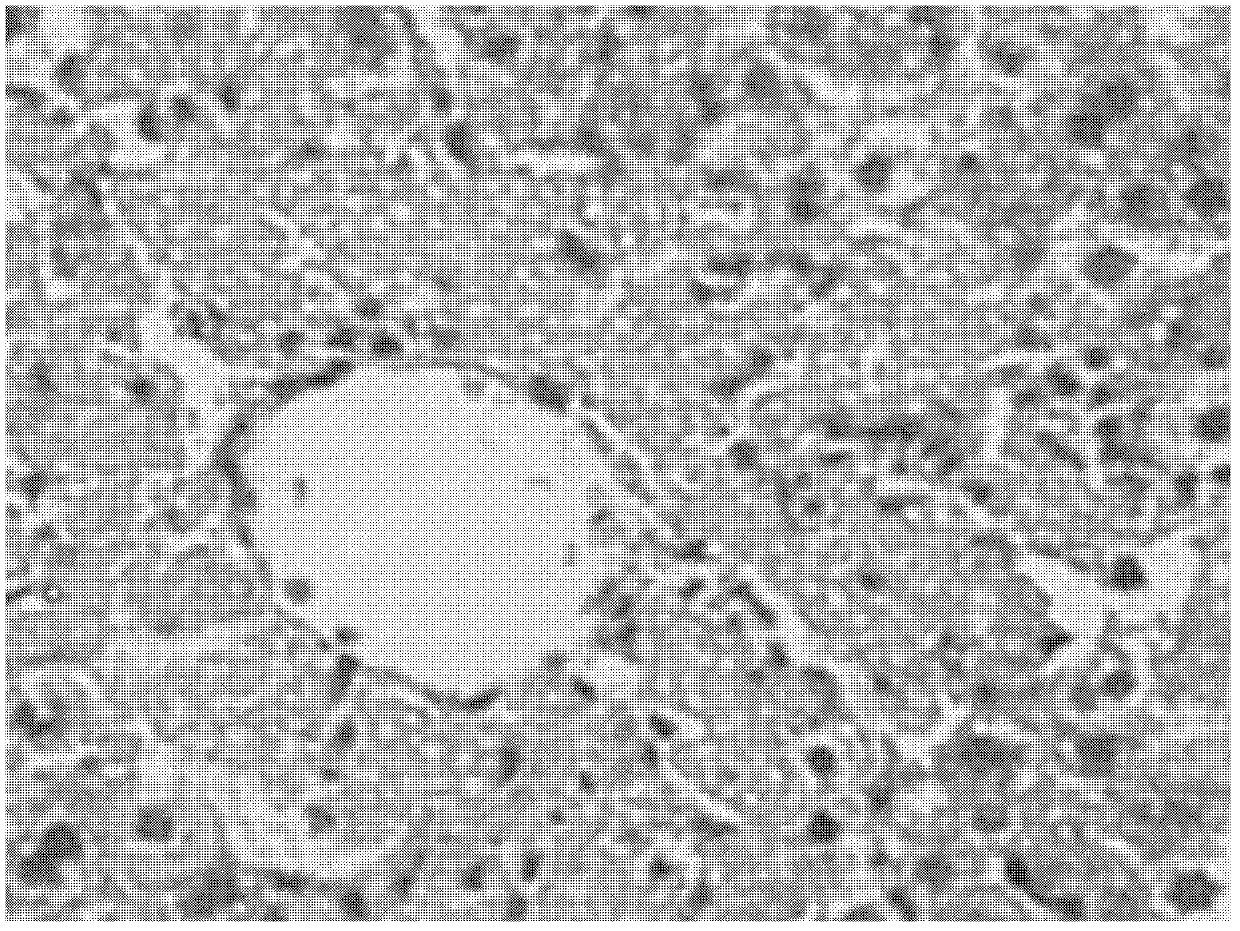
[0083] Example 2. The Effect of the Pharmaceutical Composition on the Fatty Liver Model of Mouse Acute Ethionine Liver Injury
[0084] 1. Experimental method
[0085]Male mice of Kunming species were randomly divided into 6 groups according to body weight, 12 in every group, respectively normal group, model group, positive drug ursodeoxycholic acid capsule group, pharmaceutical composition (prepared according to embodiment six) low, medium , high dose group (0.5g drug / kg body weight, 1g drug / kg body weight, 2g drug / kg body weight). The test product group was administered by intragastric administration, 1 time / day, the normal group and the model group were given equal-volume distilled water, and the volume of intragastric administration was 0.2ml / 10g body weight, and the administration was continued for 10 days; on the 10th day, except for the blank group, The rest of the animals were gavaged with 0.2% ethionine aqueous solution, and after fasting for 24 hours, the eyeballs of...
Embodiment 3
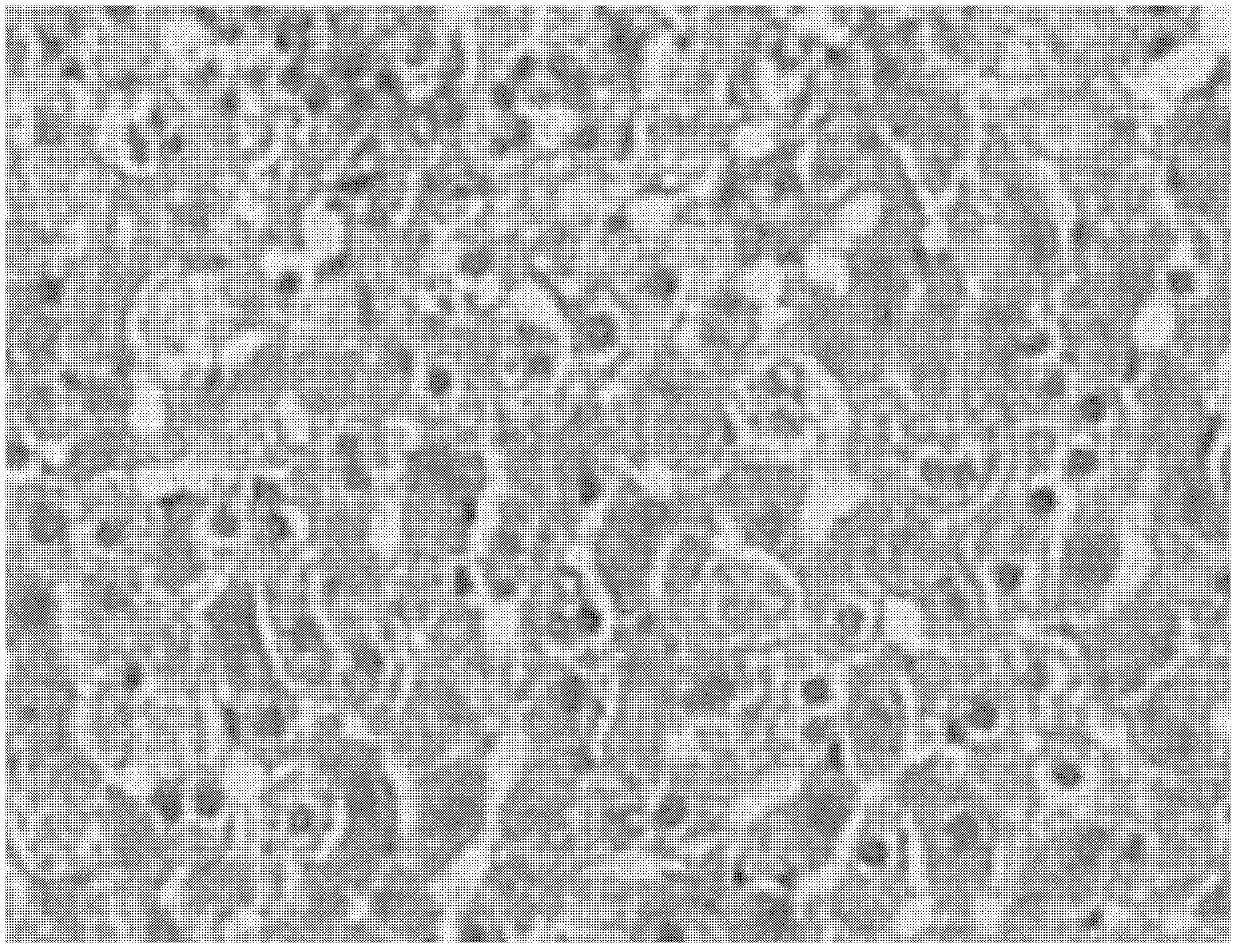
[0115] Example 3. Effect of the pharmaceutical composition on the acute alcoholic fatty liver model in mice
[0116] 1. Experimental method
[0117] Male mice of Kunming species were randomly divided into 6 groups according to body weight, 14 in every group, respectively normal group, model group, positive drug ursodeoxycholic acid capsule group, pharmaceutical composition (prepared according to embodiment six) low, medium , high dose group (0.5g drug / kg body weight, 1g drug / kg body weight, 2g drug / kg body weight). Except for the blank group, all other groups were given Luzhou Laojiao liquor with an alcohol content of 52% by intragastric administration, with a volume of 12ml / kg body weight, once a day, for 17 consecutive days. -Gavage administration started at 5 hours, the test product group was given the corresponding drug by gavage, 1 time / day, the blank group and the model group were given equal volumes of distilled water, the volume of gavage was 0.2ml / 10g body weight, co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com