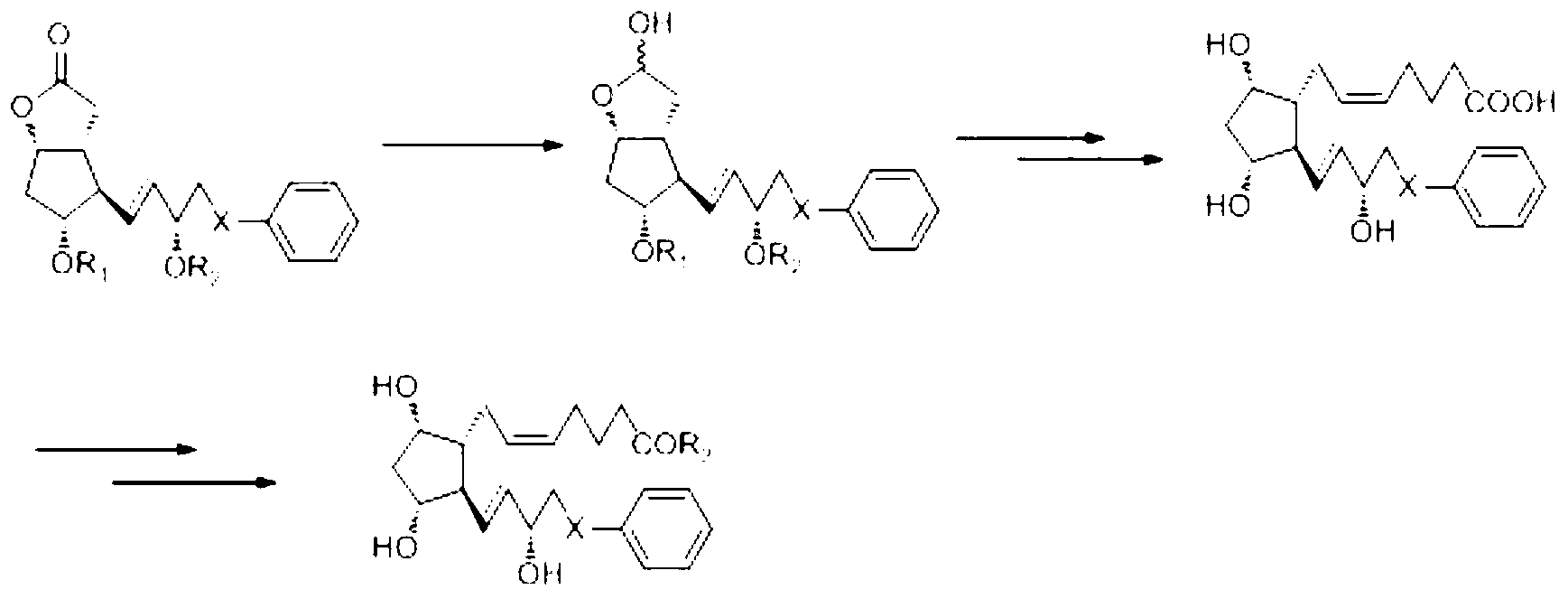
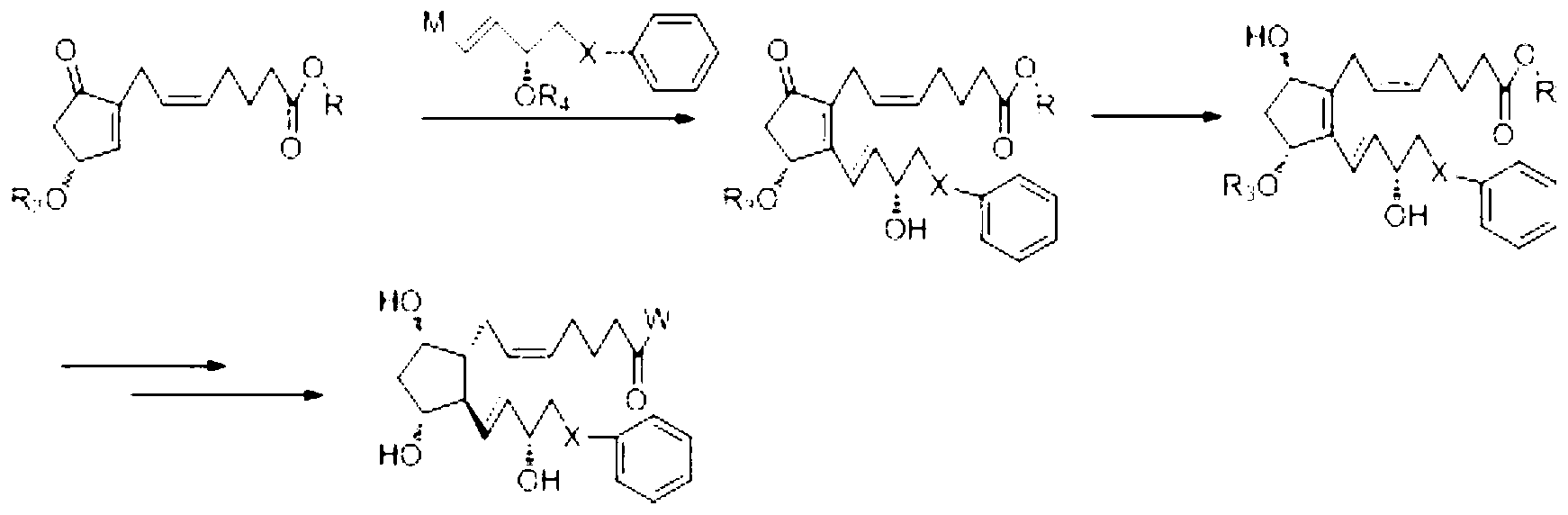
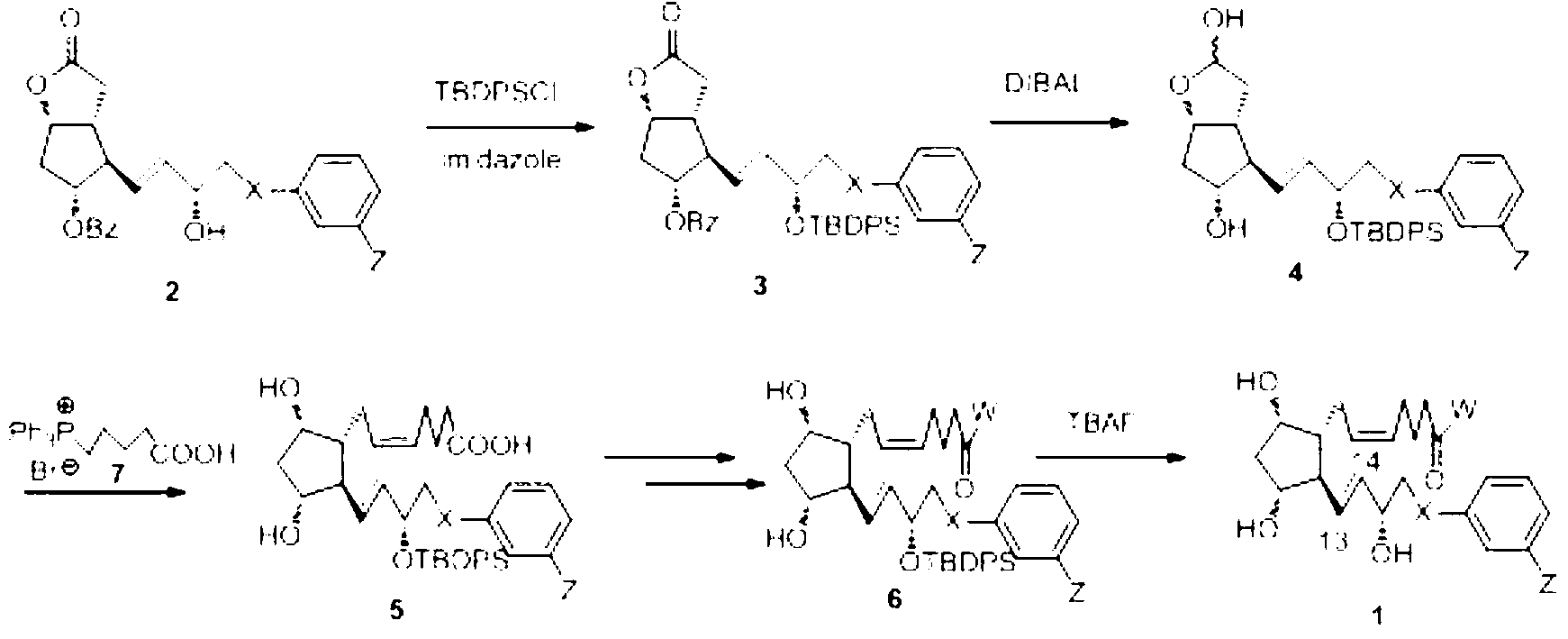
Novel method for synthetizing prostaglandin analogue
A technology of analogues and prostaglandins, which is applied in the field of synthesizing prostaglandin analogues, can solve problems such as separation difficulties, increased ratio of 5,6-trans isomers, and decreased yield, so as to enhance UV absorption and reduce extraction times , to facilitate the effect of central control monitoring
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (3aR,4R,5R,6aS)-4-((S,E)-3-(tert-butyldiphenylsilyloxy)-5-phenylpent-1-en-1-yl)-2-oxo Hexahydro-2H-cyclopenta[b]furan-5-ylbenzoate (3a)
[0041] Add 10 grams of compound 2a, 90 milliliters of dichloromethane, 5 grams of imidazole, 0.25 grams of DMAP into a 1L three-necked flask, and cool down to 0-10 degrees under nitrogen protection, and dropwise add a mixture of 10.1 grams of TBDPSCl and 30 milliliters of dichloromethane, about The addition was completed in 10 minutes, and a white insoluble substance was formed. Stir at about 20 degrees for 10 hours, TLC (Rf=0.9, toluene:EtOAc3:2) detected that the reaction was complete, washed twice with brine, dried over anhydrous sodium sulfate, and filtered to obtain compound 3a The solution was used directly for subsequent reactions.
[0042] A small amount was concentrated and then chromatographed on a silica gel column (eluent: dichloromethane:n-hexane 1:1) to obtain the pure product.
[0043] 1 H-NMR (CDCl 3 ,300MHz)δ(ppm)...
Embodiment 2
[0045] (3aR,4R,5R,6aS)-4-((S,E)-3-(tert-butyldiphenylsilyloxy)-5-phenylpent-1-en-1-yl)hexahydro-2H- Cyclopentano[b]furan-2,5-diol (4a)
[0046] Add 140 milliliters of dichloromethane to the solution of Example 1, lower the temperature to -60 degrees under nitrogen protection, add 130 milliliters of 25% DIBAL toluene solution dropwise, and finish adding in about 60 minutes. Keep stirring at -60 degrees for 1.5 hours, slowly Add 70 ml of water to destroy excess DIBAL, filter to remove aluminum hydroxide, and concentrate the filtrate. The residue is subjected to silica gel column chromatography (dichloromethane / methyl tert-butyl ether 1:1) to obtain compound 4a, 15.4 g.
[0047] 1 H-NMR (CDCl 3 ,300MHz)δ(ppm)1.05(s,9H),1.64-2.05(m,6H),2.10-2.29(m,2H),2.58(m,2H),3.60(m,1H),4.19(m, 1H),4.46-4.55(m,1H),5.02(m,1H),5.46-5.62(m,2H),7.09(m,2H),7.16(m,1H),7.22-7.25(m,2H) ,7.34-7.45(m,6H),7.66(m,4H).
Embodiment 3
[0049] (Z)-7-((1R,2R,3R,5S)-2-((S,E)-3-(tert-butyldiphenylsiloxy-5-phenylpent-1-en-1-yl )-3,5-dihydroxycyclopentyl)hept-5-enoic acid (5a)
[0050] Add 50 g of compound 7 (4-carboxybutyl-triphenylphosphine bromide) and 150 ml of anhydrous THF to a 500 ml three-necked flask, add 25.5 g of potassium tert-butoxide in batches under nitrogen protection, stir for 30 minutes, and cool down To 0 to 10 degrees, dropwise add a solution of 14.5 grams of 4a and 25 milliliters of THF, about 20 minutes to complete the addition, maintain 0 to 10 degrees and stir for 1 hour. Naturally rise to room temperature and stir for 10 to 20 hours. Remove most of the solvent under reduced pressure , the residue was dissolved in 300 ml of aqueous solution with 0.6 g of potassium carbonate, the aqueous layer was adjusted to PH=4-5 with 1N hydrochloric acid, 200 ml of MTBE was added, filtered, and the filter cake was rinsed twice with MTBE. The filtrate was separated and the organic phase was combined , an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com