Method for determining contents of cystine, cysteine and salt thereof in amino acid injection
A cysteine and determination method technology, which is applied in the field of content determination of cystine, cysteine and its salts, can solve problems affecting analysis sensitivity, content analysis interference, expensive reagents, etc., and save detection time , strong specificity and low price effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] The present invention will be further described below in conjunction with specific examples.
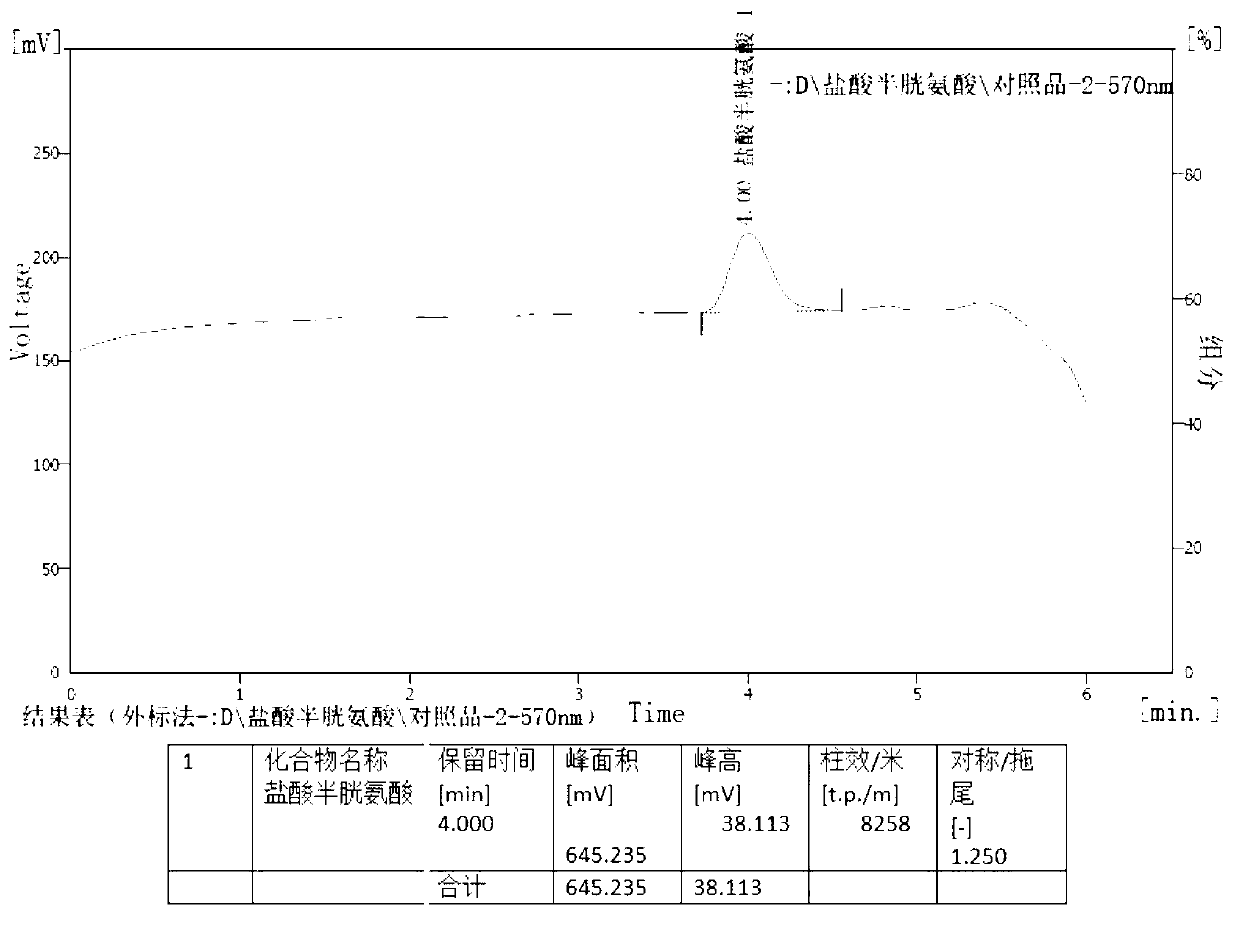
[0030] Determination of content of cysteine hydrochloride and its salts, cystine and its salts in amino acid injection
[0031] 1. Prepare the required solution:
[0032] Preparation of special mobile phase A6 solution: Take 12.0g of trisodium citrate dihydrate, 7.6g of sodium chloride, 6.0g of citric acid, 1.0g of boric acid, 120ml of methanol, 7.78ml of 37% hydrochloric acid, and 0.1ml of caprylic acid, and place them in a 1000ml measuring bottle. Add water to the volume and adjust the pH to 2.7;
[0033] Prepare a special sodium hydroxide regeneration solution: take 18.0g of sodium hydroxide and put it in a 1000ml measuring bottle, add water to make up to the mark;
[0034] Prepare potassium-sodium buffer solution (pH5.51): Take 272.0g of sodium acetate trihydrate and 196.0g of potassium acetate, add them to 400ml of water, stir while adding, add 200ml of acetic acid at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com