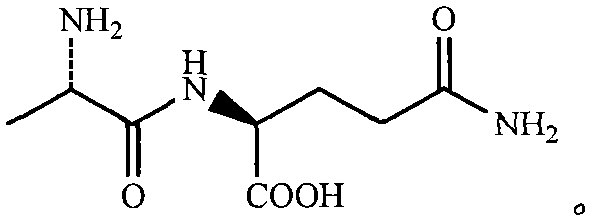
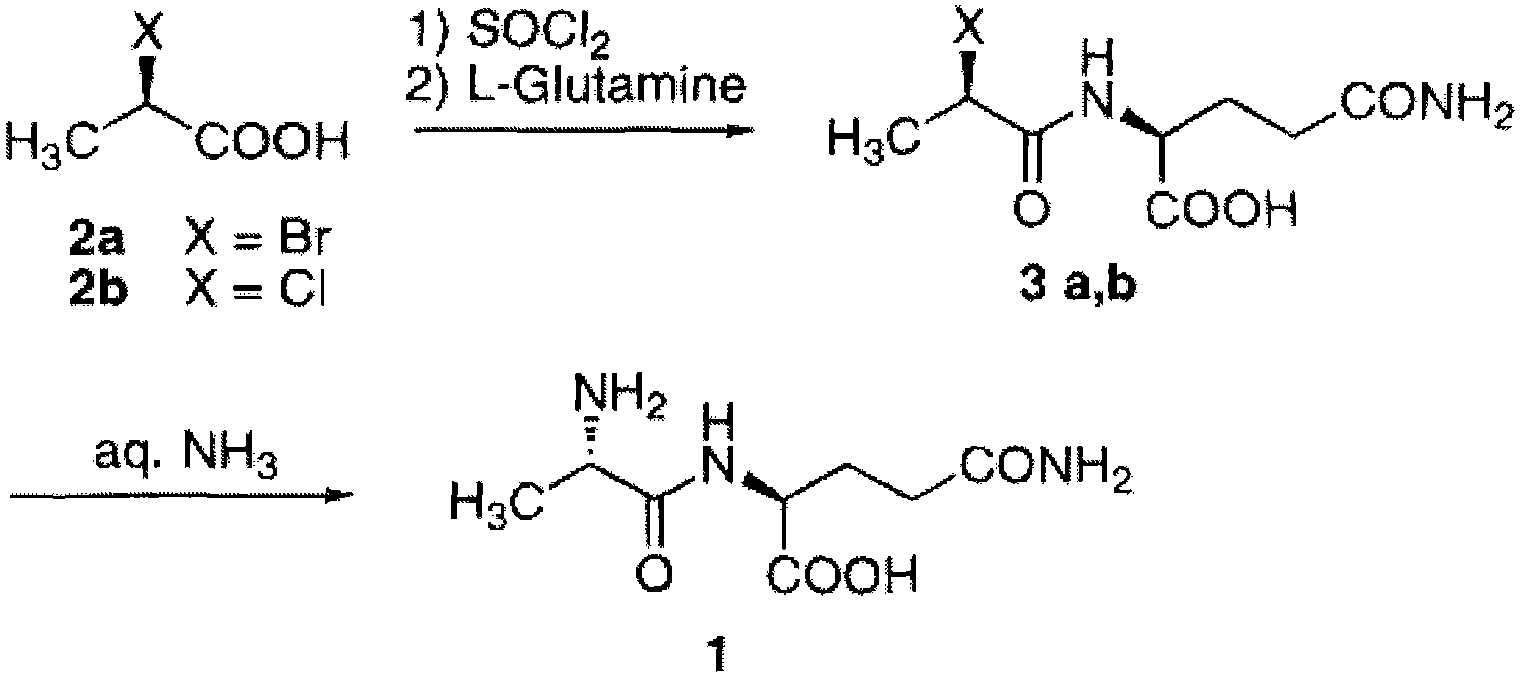
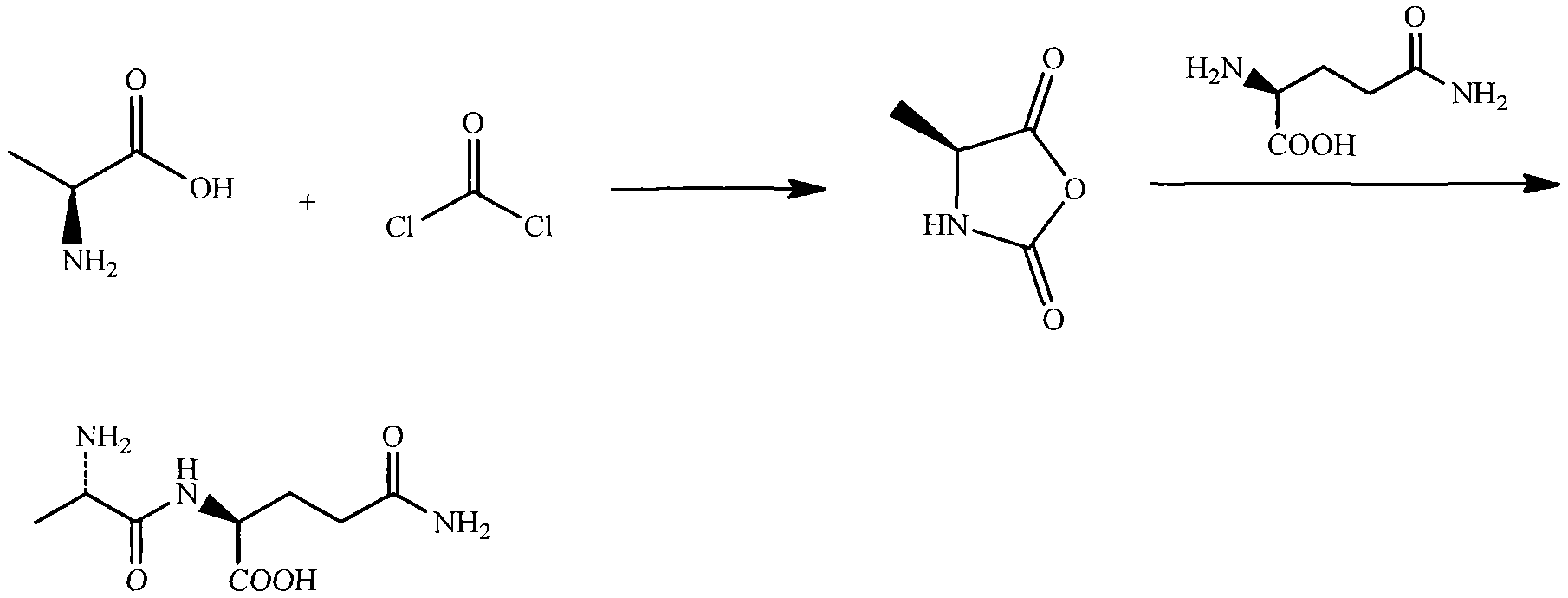
N(2)-L-alanyl-L-glutamine synthesis method
A technology of glutamine and synthetic methods, applied in the direction of peptides, etc., can solve the problems of cumbersome operation steps, little application prospect, and high price, and achieve the effect of reducing cost, low cost, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1. Synthesis of pyruvyl-L-glutamine
[0037]Add 73g (0.5mol) L-glutamine to 500mL water, add 10% NaOH dropwise at 0°C to adjust the pH to 10, keep the temperature, add 68.9g (0.65mol) pyruvate chloride dropwise, and keep the pH during the dropwise addition Value is about 10. After the dropwise addition, react at 0-5°C for 30 minutes, rise to room temperature and continue the reaction for 4 hours, then stop the reaction. Under ice-bath conditions, add 2mol / L HCl dropwise to adjust the pH to 1-2, and stand overnight at 0-5°C, a large amount of white solids precipitate, filter, wash with a small amount of ice water, and dry in vacuo to obtain 77.8g of white crystals, with a yield of 74 %, m.p.83-85°C. 1 H-NMR (CDCl 3 ), δ: 8.03(s, 1H), 7.16(s, 2H), 4.56(t, 1H), 2.17(s, 3H), 2.12(m, 2H), 2.05(t, 2H). EI-MS m / z: 216.
[0038] 2. Synthesis of N(2)-2'-hydroxyimino-propionyl-L-glutamine and
[0039] Dissolve 54g (0.25mol) of pyruvic glutamine in 150mL of ethanol, add drop...
Embodiment 2
[0043] 1. Synthesis of pyruvyl-L-glutamine
[0044] Add 73g (0.5mol) L-glutamine to 600mL water, add 5% KOH dropwise at 2°C to adjust the pH to 11, keep the temperature, add 0.8mol pyruvate chloride dropwise, and keep the pH at about 11 during the dropwise addition . After the dropwise addition, react at 0-3°C for 40 minutes, rise to room temperature and continue the reaction for 5 hours, then stop the reaction. Under the condition of ice bath, add 1mol / LH dropwise 2 SO 4 Adjust the pH to 2-3, and let it stand overnight at 0-3°C. A large amount of white solids precipitated out. Filter, wash with a small amount of ice water, and dry in vacuo to obtain 77.8 g of white crystals, yield 76%, m.p.83-85°C. 1 H-NMR (CDCl 3 ), δ: 8.03(s, 1H), 7.16(s, 2H), 4.56(t, 1H), 2.17(s, 3H), 2.12(m, 2H), 2.05(t, 2H). EI-MS m / z: 216.
[0045] 2. Synthesis of N(2)-2'-benzyloxyimino-propionyl-L-glutamine
[0046] Dissolve 54g (0.25mol) of pyruvylglutamine in 150mL of methanol, add 36.9g (0.3m...
Embodiment 3
[0050] 1. Synthesis of pyruvyl-L-glutamine
[0051] Add 73g (0.5mol) of L-glutamine to 400mL of water, add 8% NaOH dropwise at 1°C to adjust the pH to 9, keep the temperature, add 1mol of pyruvate chloride dropwise, and keep the pH at about 9 during the dropwise addition. After the dropwise addition, react at 5-10°C for 50 minutes, rise to room temperature and continue the reaction for 6 hours, then stop the reaction. Under ice-bath conditions, add 3mol / L HCl dropwise to adjust the pH to 2-3, and stand overnight at 5-10°C, a large amount of white solids precipitated, filtered, washed with a small amount of ice water, and dried in vacuo to obtain 77.8g of white crystals with a yield of 72 %, m.p.83-85°C. 1 H-NMR (CDCl 3 ), δ: 8.03(s, 1H), 7.16(s, 2H), 4.56(t, 1H), 2.17(s, 3H), 2.12(m, 2H), 2.05(t, 2H). EI-MS m / z: 216.
[0052] 2. Synthesis of N(2)-2'-allyloxyimino-propionyl-L-glutamine
[0053] 54g (0.25mol) of pyruvyl glutamine was dissolved in 200mL of isopropanol, and 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com