Preparation and application of chromotropic acid intercalated hydrotalcite composite material
A composite material and hydrotalcite technology, applied in water/sewage treatment, adsorption water/sewage treatment, water/sludge/sewage treatment, etc., to achieve high selectivity and high effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] 1) Take Chromotropic acid and use 200ml to remove CO 2 deionized water to prepare a concentration of 0.3mol / L Chromotropicacid solution, adjust the pH value to 7 with 2M NaOH solution; then add 0.06molZn(NO 3 ) 2 ·6H 2 O, 0.02molAl(NO 3 ) 3 9H 2 O;
[0020] 2) Take 9.60g NaOH and use 200ml to remove CO 2 deionized water to prepare alkaline solution;
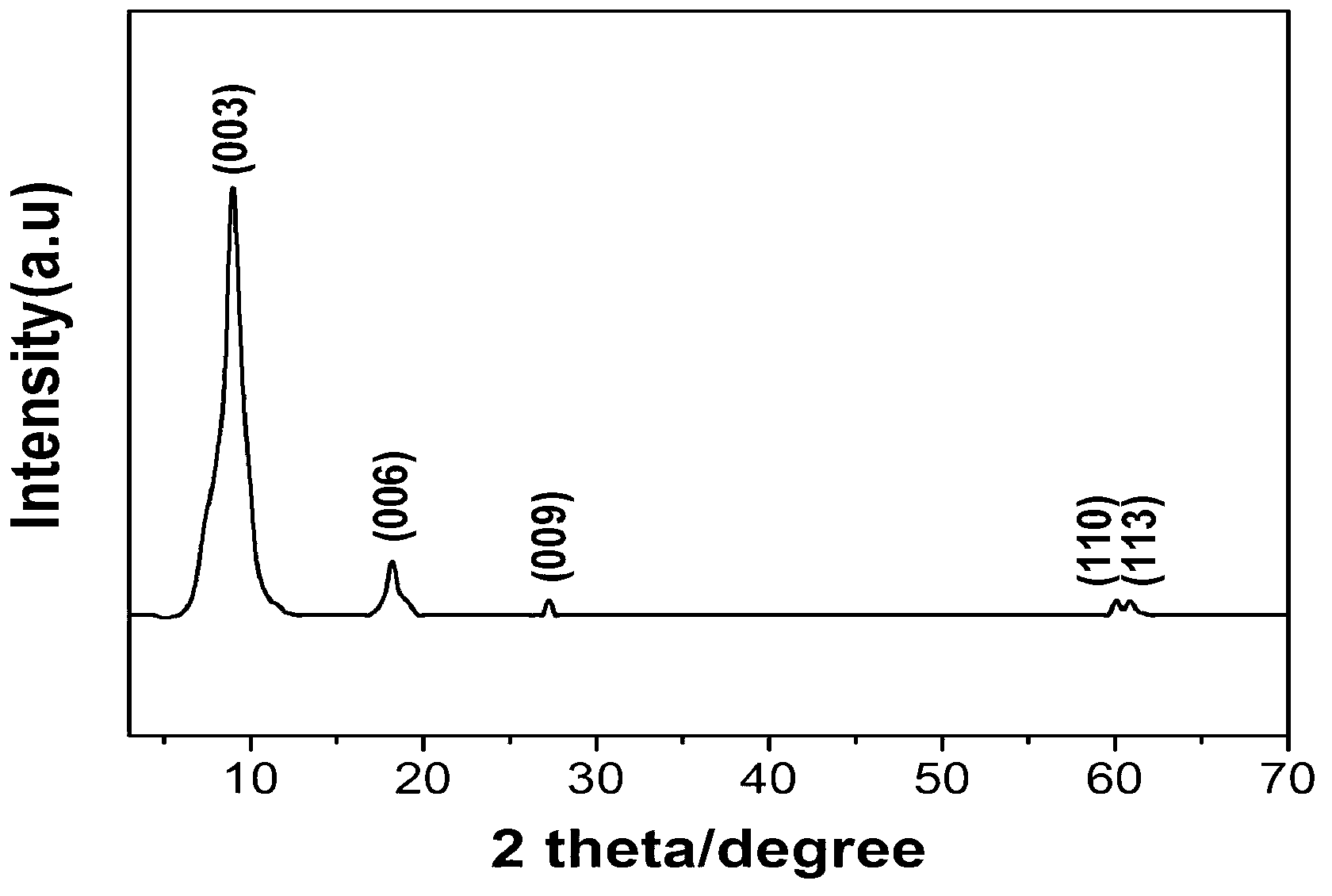
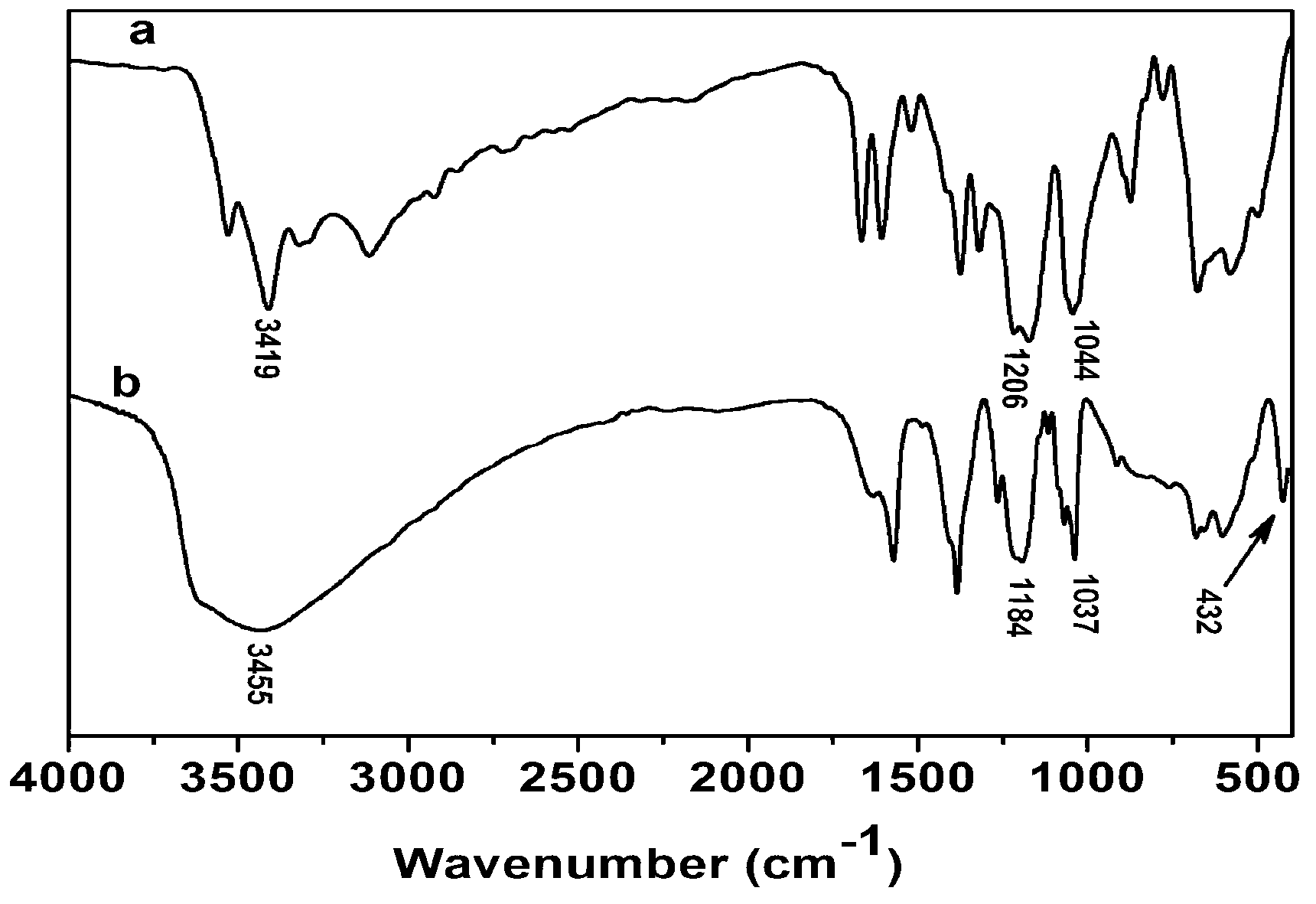
[0021] 3) Add the solution prepared in step 1) and step 2) into the colloid mill with a rotation speed of 3000rpm and mix for 2 minutes, and the obtained suspension is placed in an inert atmosphere N 2 Reflux aging at 97°C for 8 hours under protection, the product was centrifuged and washed 4 times, and dried in vacuum to obtain the Chromotropicacid intercalated hydrotalcite composite material Zn 3 Al-CTA-LDH, its chemical formula is [Zn 2+ 1-x al 3+ x (OH) 2 ] x+ (C 10 h 6 o 8 S2 2- ) x / 2 ﹒ 3H 2 O, x=0.25.
Embodiment 2
[0023] 1) Take Chromotropic acid and use 200ml to remove CO 2 deionized water to prepare a concentration of 0.8mol / L Chromotropicacid solution, adjust the pH value to 7 with 2M NaOH solution; then add 0.06molMg(NO 3 ) 2 ·6H 2 O, 0.02molAl(NO 3 ) 3 9H 2 O;
[0024] 2) Take 9.6g NaOH and use 200ml to remove CO 2 deionized water to prepare alkaline solution;
[0025] 3) Add the solution prepared in step 1) and step 2) into the colloid mill with a rotation speed of 3000rpm to mix for 4 minutes, and the obtained suspension is placed in an inert atmosphere N 2 Reflux aging at 97°C for 10 h under protection, the product was centrifuged and washed 3 times, and dried in vacuum to obtain the Chromotropicacid intercalated hydrotalcite composite material Mg 3 Al-CTA-LDH, its chemical formula is [Mg 2+ 1-x Al 3+ x (OH) 2 ] x+ (C 10 h 6 o 8 S 2 2- ) x / 2 ﹒ 5H 2 O, x=0.25.
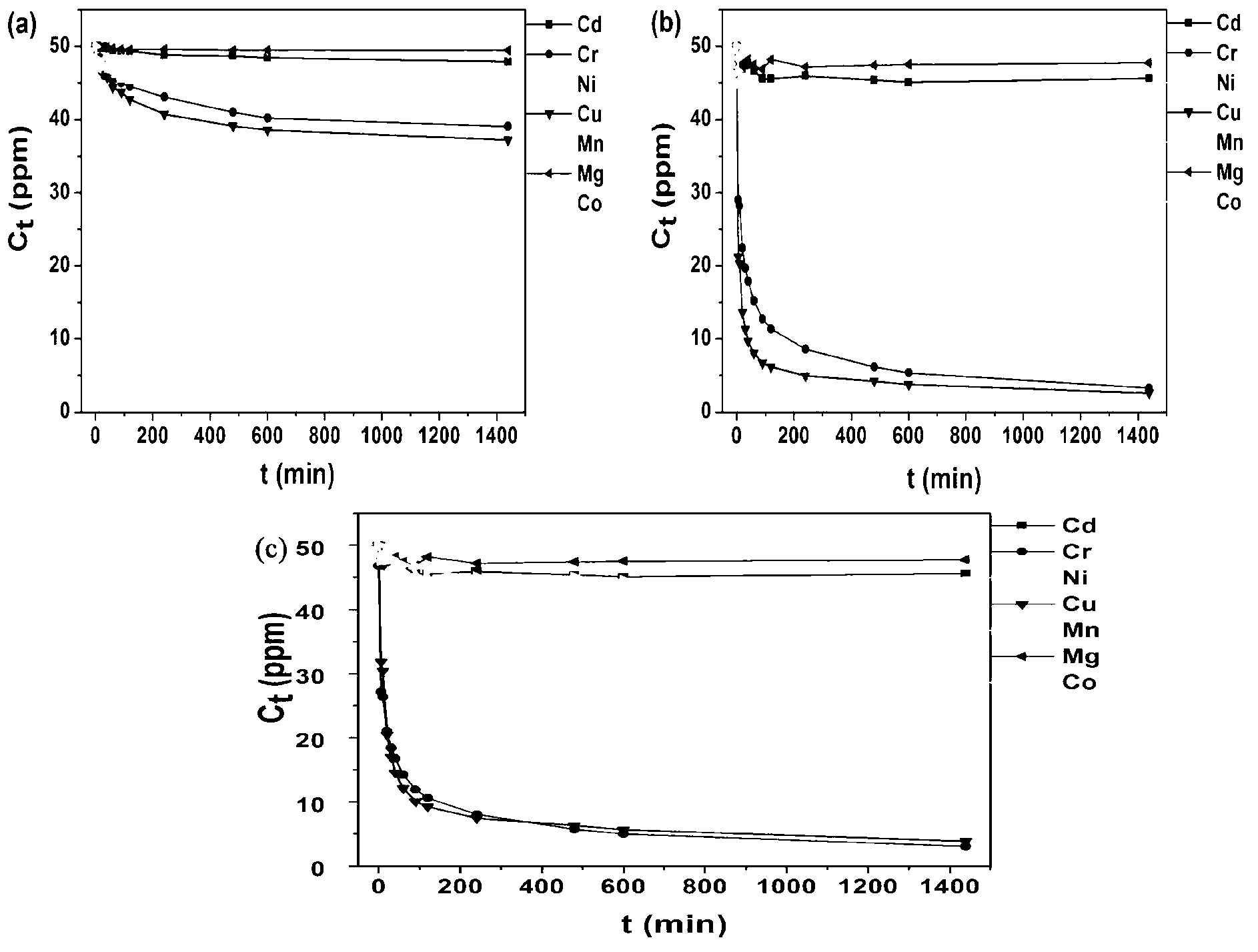
[0026] In order to verify the adsorption performance of the Chromotropicacid intercalated hydrot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com