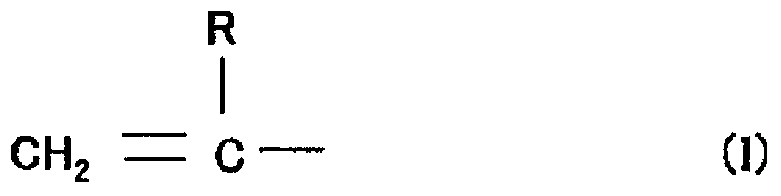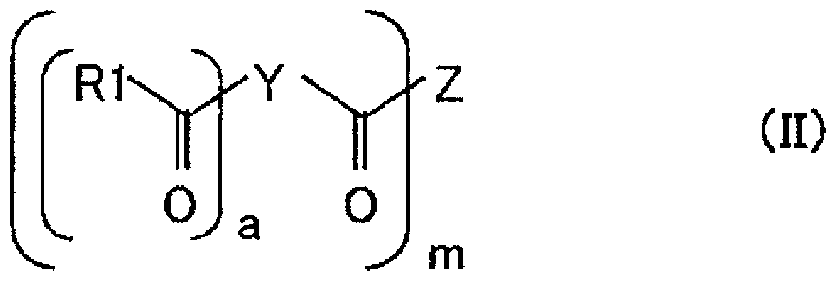Polyol composition for production of polyurethane resins, and polyurethane resin producing process using same
A technology of polyol composition and polyurethane resin, which is applied in the field of polyol (polyol) composition, can solve the problems of reduced combustion resistance and extremely reduced hardness of polyurethane foam, and achieves the effect of excellent flame retardancy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
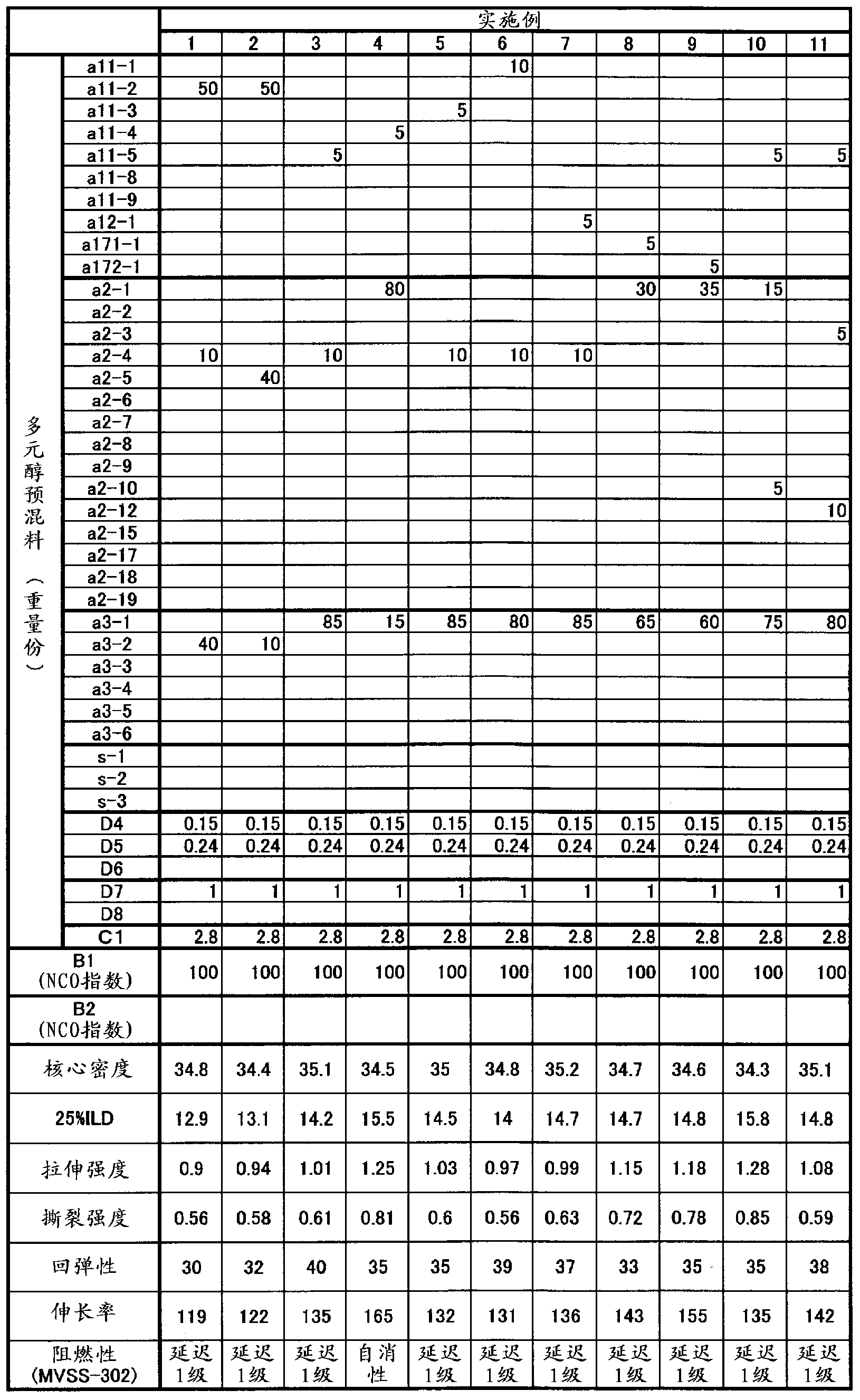
[0167] The present invention will be further described by the following examples, but the present invention is not limited to these examples.
[0168] The raw materials of the foamed polyurethane resin in the following Examples and Comparative Examples are as follows.
[0169] (1) Compounds with active hydrogen-containing groups and vinyl polymerizable functional groups
[0170] (A11-1) Ethylene containing glycerol PO adduct (number average molecular weight of 1500, hydroxyl value of 112.2) and acrylic acid with a hydroxyl value of 35 and a vinyl polymerizable functional group in the molecule of 1.2 mmol / g Active hydrogen compounds based on polymerizable functional groups
[0171] (A11-2) Ethylene containing pentaerythritol PO adduct (number-average molecular weight of 1000, hydroxyl value of 224.4) and acrylic acid, which has a hydroxyl value of 48 and a vinyl polymerizable functional group in the molecule of 2.6 mmol / g Active hydrogen compounds based on polymerizable functional gro...
manufacture example 1
[0202] Production Example 1 [Production of Strength Improver (a2-1)]
[0203] Put 1 mole of glycerin PO adduct (number average molecular weight of 1700, hydroxyl value of 99.0), 6 moles of phthalic anhydride, and alkali catalyst (N-ethyl) into a stainless steel autoclave equipped with a stirring device and a temperature control device. Morpholine) 0.020 mol, reacted for 1 hour at 0.20 MPa and 120±10°C in a nitrogen atmosphere for half-esterification. Then, while controlling the conditions at 120±10°C and the pressure at 0.50 MPa or less, 6 mol of EO was added dropwise over 5 hours, and then aged at 120±10°C for 1 hour. After the maturation, the alkali catalyst was removed under reduced pressure at 10 KPa for 1 hour to obtain a strength improver (a2-1). The values of (a2-1) are as follows. Hydroxyl value (mgKOH / g) = 59.0, aromatic ring concentration (mmol / g) = 2.1.
manufacture example 2
[0204] Production Example 2 [Production of Strength Improver (a2-2)]
[0205] Into the same autoclave as in Production Example 1, 1 mol of glycerin PO adduct (number average molecular weight of 3000, hydroxyl value of 56.1), 3 mol of phthalic anhydride, and base catalyst (N-ethylmorpholine) 0.020 were charged Mol, the reaction was carried out at 0.20 MPa and 120±10°C for 1 hour in a nitrogen atmosphere for half-esterification. Then, while controlling the conditions at 120±10°C and the pressure at 0.50 MPa or less, 3 mol of EO was added dropwise over 5 hours, and then aged at 120±10°C for 1 hour. After the maturation, the alkali catalyst was removed under reduced pressure at 10 KPa for 1 hour to obtain a strength improver (a2-2). The values of (a2-2) are as follows. Hydroxyl value (mgKOH / g) = 47.1, aromatic ring concentration (mmol / g) = 0.8.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of unsaturation | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com