Method for synthesizing multi-substituted quinoline compound
A quinoline-based, multi-substitution technology, applied in the field of synthesis of drug molecules and their analogs, can solve the problems of heavy metal drug residues, unsuitable for wide application, and difficult separation and recovery of transition metal catalysts, achieving easy separation and low dosage Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] One, take synthetic 2,3-diphenylquinoline-3-carboxylic acid ethyl ester as example production steps:
[0020] 1. Add 1g of raw material 3-(1,2-diphenylethanon-2-yl)indol-2-one, 50mL of absolute ethanol, 50mL of chloroform and 0.01g of p-toluenesulfonic acid into a 250mL round bottom flask .
[0021] 2. The above mixture was heated to 70°C, stirred and kept for 30 minutes.
[0022] 3. Pour the solution obtained after the reaction in step 2 into 150 mL of water to precipitate solids.
[0023] 4. The above solid was filtered out without further purification to obtain the final compound—ethyl 2,3-diphenylquinoline-3-carboxylate.
[0024] 5. Reaction formula:
[0025]
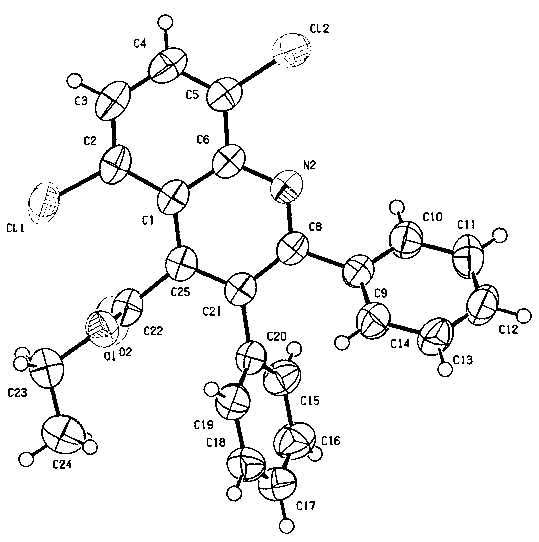
[0026] 2. Verification:
[0027] Adopt the structural diagram of the product that the inventive method obtains, as figure 1 as shown in figure 2 The spectra shown are verified as follows:
[0028]
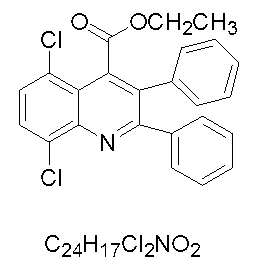
[0029] 5,8-Dichloro-2,3-diphenylquinoline-4-carboxylic acid ethyl ester
[0030] White solid, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com