Nitramine explosive and preparation method thereof
The technology of nitramine explosive and trinitro group is applied in the field of explosive synthesis, which can solve the problems of long reaction time and the like, and achieve the effects of less environmental damage, simple product post-processing and simple reaction steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] Nitramine explosive 1,3,5-trinitro-2,3-dihydro-1 of the present invention H -The preparation method of imidazo[4,5-b]pyridin-2-one, comprising the following steps:
[0029] In the first step, 2,3-diaminopyridine and urea are put into the reactor, and the ring-closing reaction of 2,3-diaminopyridine and urea is realized at high temperature. After the reaction, the mixture is extracted with boiling ethanol and filtered A dark brown solid was obtained;
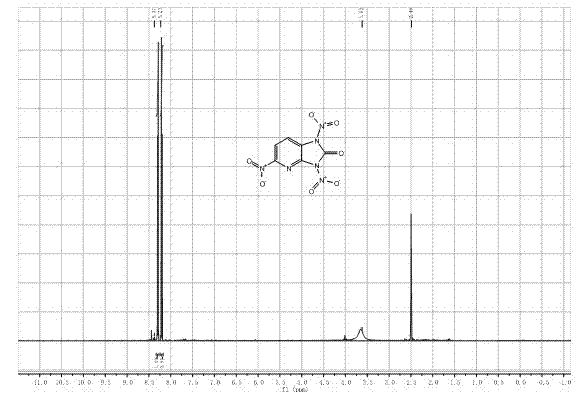
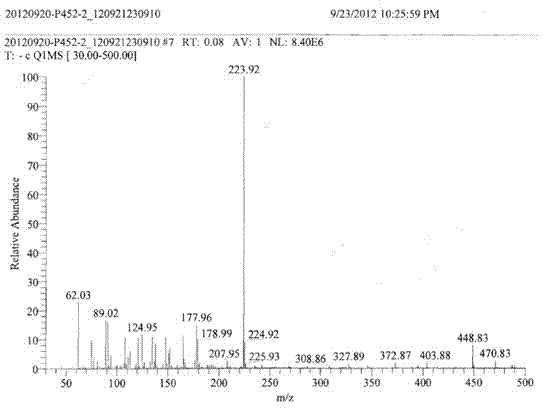
[0030] In the second step, the black solid obtained in the first step is subjected to a nitration reaction in a nitration system. After the reaction, the mixture is poured into crushed ice, filtered with suction, and the filter cake is washed with water until it is neutral, and dried to obtain the product, which is identified as 1,3,5-Trinitro-2,3-dihydro-1 H - imidazo[4,5-b]pyridin-2-one.
[0031] Among them, in the first step, the molar ratio of 2,3-diaminopyridine to urea is 1:1~1:3.3, the ring closing temperatur...
Embodiment 1
[0035] Add 763.9 mg (7.0 mmol) of 2,3-diaminopyridine and 1.40 g (23.3 mmol) of urea into a 50 mL one-necked flask, mix well, and heat to 140 °C under reduced pressure for 1 h. The cooled reaction mixture was extracted with boiling ethanol (5*6 mL), and the crystals were collected by filtration. The filtrate was refrigerated in a refrigerator, and solids precipitated after overnight, filtered by suction, and dried to obtain 2,3-dihydro-1 H - Imidazo[4,5-b]pyridin-2-one, yield 95%.
[0036] m.p.273~275℃;
[0037] 1 H NMR (DMSO- d 6 , 500 MHz): δ 6.93(dd, 1H), 7.21(dd, 1H), 7.85(dd, 1H), 10.81(s, 1H), 11.28(s, 1H);
[0038] Elemental Analysis: C 6 h 5 N 3 O, Calculated: C, 53.33; H, 3.73; N, 31.10; Found: C, 53.26; H, 3.92; N, 31.15;
[0039] MS (ESI) m / z: 136.03(M+H).
Embodiment 2
[0041] Add 763.9 mg (7.0 mmol) of 2,3-diaminopyridine and 1.40 g (23.3 mmol) of urea into a 50 mL one-necked flask, mix well, and heat to 120 °C under reduced pressure for 0.2 h. The cooled reaction mixture was extracted with boiling ethanol (5*6 mL), and the crystals were collected by filtration. The filtrate was refrigerated in a refrigerator, and solids precipitated after overnight, filtered by suction, and dried to obtain 2,3-dihydro-1 H - Imidazo[4,5-b]pyridin-2-one, yield 36%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com