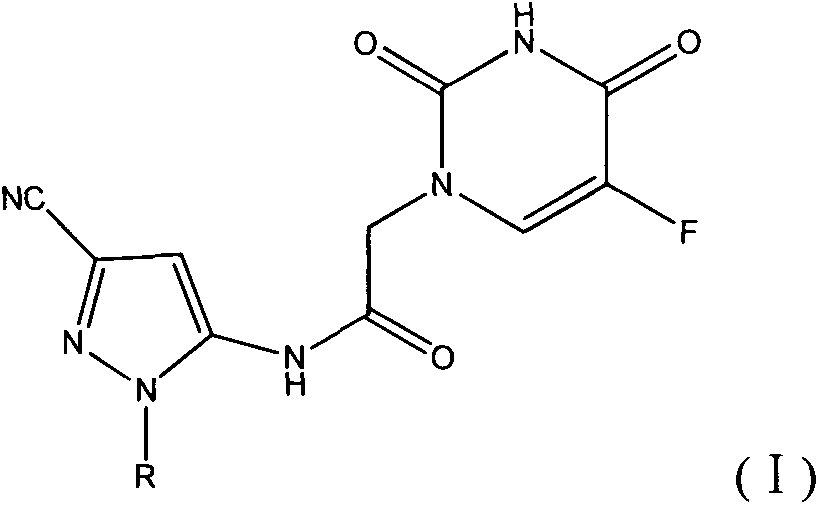
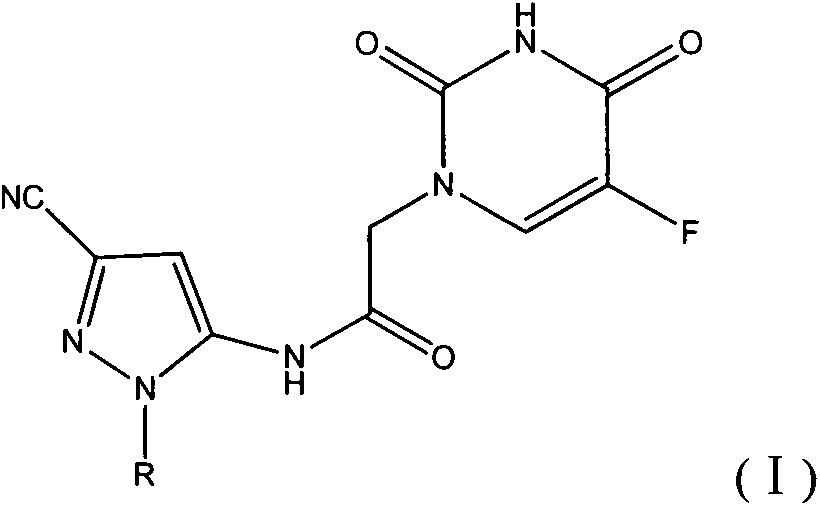
Halobenzene cyano pyrazol compound with insecticidal action as well as preparation method and application
A compound and a technology for preparing pesticides, applied in the field of pesticides, can solve problems such as low drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] This example illustrates the preparation of 5-amino-1-(4-fluorophenyl)-3-cyano-1H-pyrazole
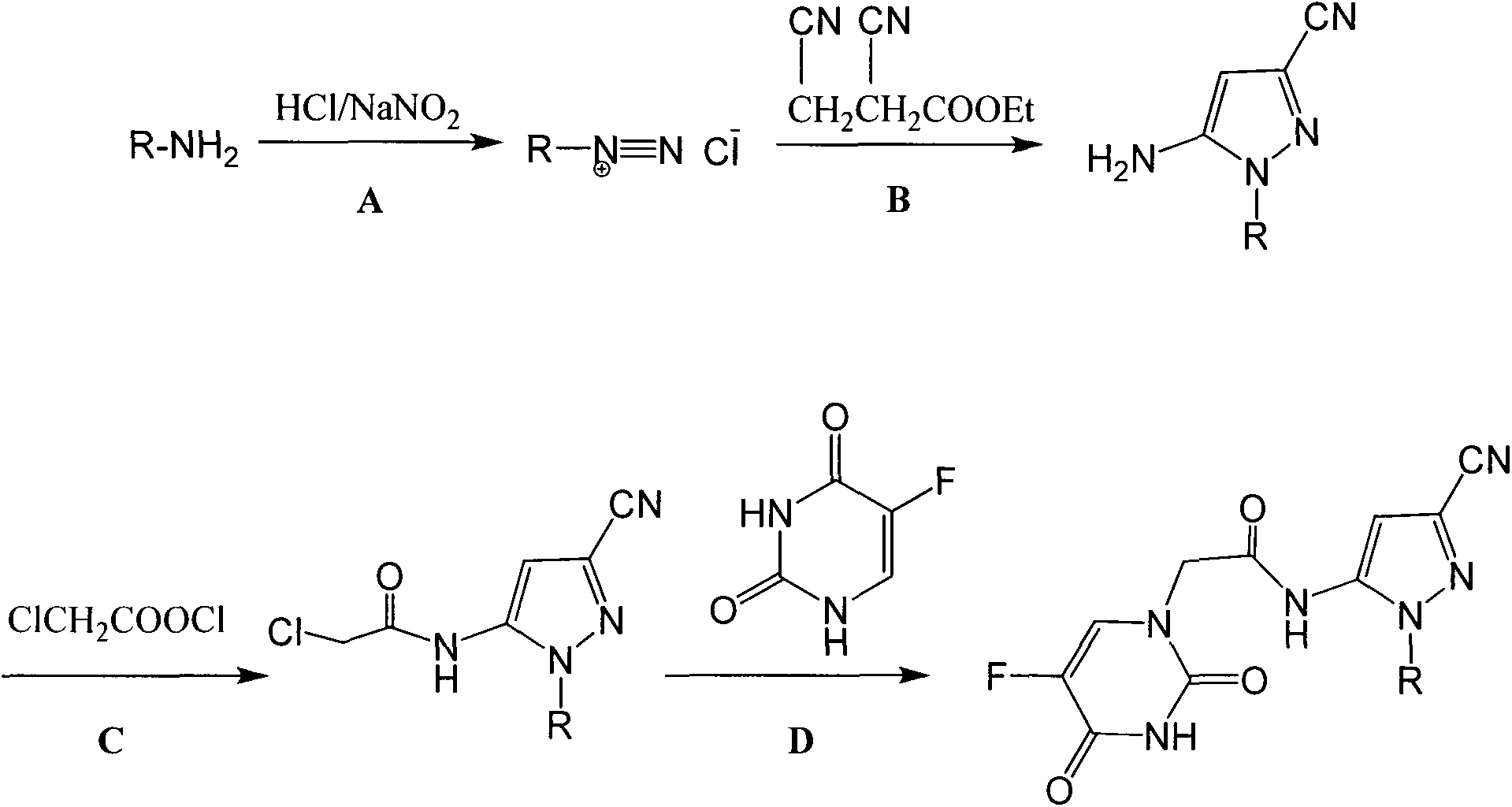
[0046] Add 0.01 mol of 4-fluoroaniline and a small amount of ethanol to a 250 ml round-bottomed three-neck flask, and add 3.0 ml (0.035 mol) of concentrated hydrochloric acid dropwise with stirring under ice-bath conditions. Dissolve 0.018mol of sodium nitrite in 10ml of water, slowly drop it into the flask, and react for 0.5h after the dropwise addition to obtain a yellow diazonium salt solution.
[0047] Add 0.01mol ethyl 2,3-dicyanopropionate into the three-necked flask, drop the prepared diazonium salt solution into the flask, and react for 2 hours after the dropwise addition. Add ammonia water, adjust the pH to 9-10, and react at room temperature for 2 hours. After the reaction was completed, it was extracted with 40ml of dichloromethane, the organic layer was washed with water (2×30mL), washed with saturated sodium chloride solution (1×40mL), dried over anhydrous magnesiu...
Embodiment 2
[0049] This example illustrates the preparation of 2-chloroN-(3-cyano-1-(4-fluorophenyl)-1H-pyrazol-5-yl)acetamide
[0050] Add 0.01mol 5-amino-1-(4-fluorophenyl)-3-cyano-1H-pyrazole and 40ml dichloromethane into a 100ml four-neck flask, stir to dissolve, add 0.015mol chlorine dropwise under ice bath Acetyl chloride. Reaction at room temperature for 2h. After the reaction, filter and recrystallize the filter cake with ethanol to obtain 2.31 g of the product. Yield 83%. Product melting point: 105-107°C.
Embodiment 3
[0052] This example illustrates the synthesis of N-(3-cyano-1-(4-fluorophenyl)-1H-pyrazol-5-yl)-2-(5-fluorouracil-1-yl)acetamide (Compound 1)
[0053] Add 0.006mol of 5-fluorouracil and 150ml of DMF into a 250ml four-neck flask equipped with mechanical stirring, reflux condenser and thermometer, raise the temperature to 100°C and stir for 1h. 50ml of 0.002mol 2-chloro N-(3-cyano-1-(4-fluorophenyl)-1H-pyrazol-5-yl)acetamide DMF solution was added dropwise, and the reaction was continued for 5h after the dropwise addition. After the reaction, the solvent was concentrated to precipitate a solid, which was filtered and dried to obtain a crude product. Column chromatography using ethyl acetate as the eluent gave 0.41 g of the final product. Yield 55.4%. Melting point: 231-232°C; 1 HNMR (300MHz, DMSO-d 6 ) δ 11.57 (s, 1H, -CO-NH-CO-), 10.23 (s, 1H, -CO-NH), 7.99 (d, J=6.7Hz, 1H, -CF=CH), 7.89 (t , J=3.5Hz, 2H, Ar-H), 7.33(t, J=5.1Hz, 2H, Ar-H), 7.15(s, 1H, =CH), 4.47(s, 2H, CH ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com