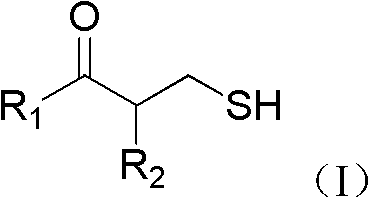
Application of 3-mercaptopropionamide compounds
A technology for mercaptopropionamide and compound, which is applied in the field of 3-mercaptopropionamide compounds, can solve problems such as high in vitro sensitivity, and achieve the effects of improving curative effect, eliminating hydrolysis, and having good drug application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
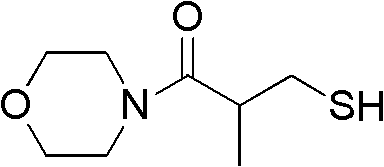
[0040] Example 1 Determination of 3-mercapto-2-methyl-1-morpholine propionamide inhibiting NDM-1 activity
[0041]
[0042] Dissolve the above-mentioned 3-mercapto-2-methyl-1-morpholine propionamide (4 mg) in 95% DMSO (211.3 μL) to prepare a solution with a concentration of 100 mM, and then place the solution in a 1.5 ml ep tube , stored at 4°C.
[0043] Then according to the above activity test method step 5 (preliminary screening of compound) and step 6 (IC of compound) 50 Determination of the value) described in the operation. When the final concentration was 1.3mM, the inhibition rate of NDM-1 reached 51.44%
Embodiment 2
[0044] Example 2 Determination of 3-mercapto-2-methyl-N-phenylpropanamide inhibiting NDM-1 activity
[0045]
[0046] Dissolve the above-mentioned 3-mercapto-2-methyl-N-phenylpropanamide (4 mg) in 95% DMSO (204.8 μL) to prepare a solution with a concentration of 100 mM, and then place the solution in a 1.5 ml ep tube , stored at 4°C.
[0047] Then according to the above activity test method step 5 (preliminary screening of compound) and step 6 (IC of compound) 50 Determination of the value) described in the operation. Then take the logarithm of the concentration of 3-mercapto-2-methyl-N-phenylpropanamide as the abscissa and the residual activity of NDM-1 as the ordinate to draw a curve, as shown in FIG. 3 . Finally, according to the curve, using GraphPad Prismversion 5.0 software to calculate, the obtained IC 50 The value is 199.50±44.53 μM.
Embodiment 3
[0048] Example 3 Determination of NDM-1 activity inhibited by N-butyl-3-mercapto-2-methylpropionamide
[0049]
[0050] Dissolve the above-mentioned N-butyl-3-mercapto-2-methylpropionamide (4 mg) in 95% DMSO (228.1 μL) to prepare a solution with a concentration of 100 mM, and then place the solution in a 1.5 ml ep tube , stored at 4°C.
[0051] Then according to the above activity test method step 5 (preliminary screening of compound) and step 6 (IC of compound) 50 Determination of the value) described in the operation. Then take the logarithm of the concentration of N-butyl-3-mercapto-2-methylpropionamide as the abscissa and the residual activity of NDM-1 as the ordinate to draw a curve, as shown in FIG. 4 . Finally, according to the curve, using GraphPad Prismversion 5.0 software to calculate, the obtained IC 50 The value is 20.65±1.24 μM.
[0052] According to the above examples, it can be known that the compounds involved in the present invention have the effect of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com