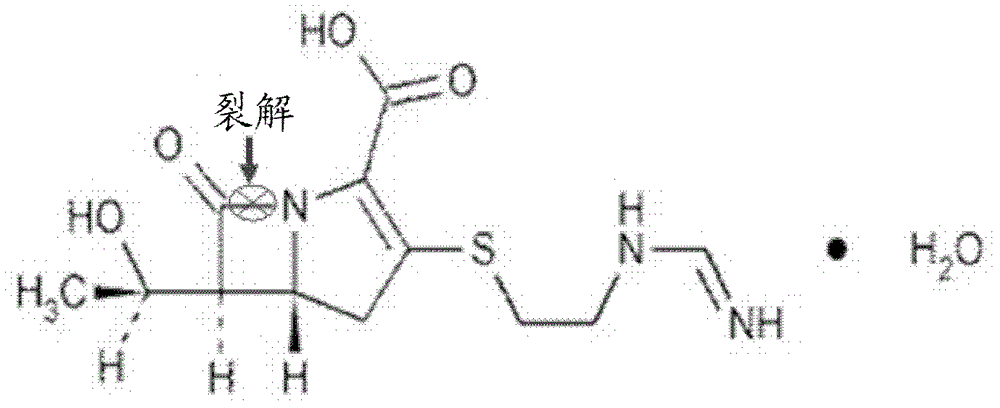
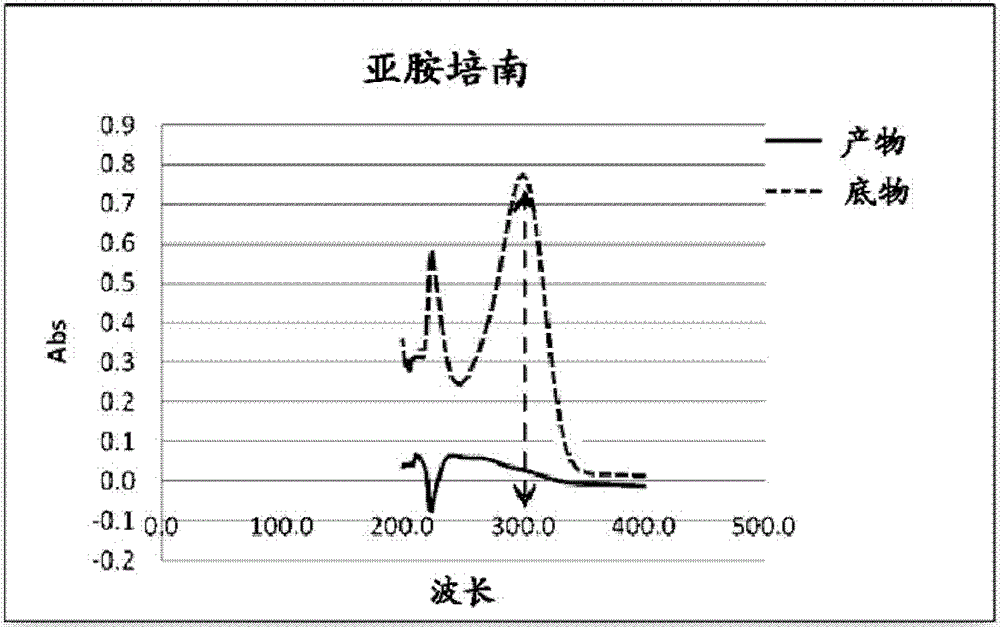
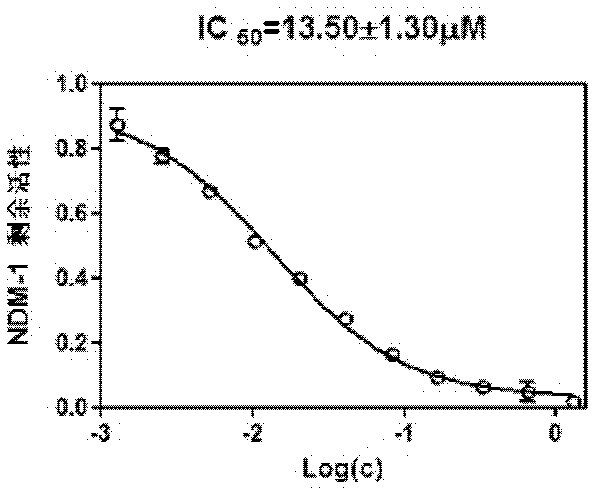Application of 3-mercaptopropionamides in inhibiting ndm-1 activity
A technology for mercaptopropionamide and compound, which is applied in the field of 3-mercaptopropionamide compounds, can solve problems such as high in vitro sensitivity, and achieve the effects of improving curative effect, relieving drug resistance, and good drug application prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1 3-mercapto-N-methoxy-N,2-dimethylpropionamide
[0054]
[0055] At room temperature (20°C-30°C), the compound 3-acetylsulfanyl-N-methoxy-N,2-dimethylpropanamide (100.15mg, 0.49mmol) obtained in Preparation 1 above was dissolved into 10ml of methanol, then added sodium bicarbonate (45.02mg, 0.54mmol), and reacted at room temperature (20°C to 30°C) for 8 hours, then spin-dried the methanol, dissolved the residue in water (15ml), and washed with petroleum ether (3*20ml) extraction, then the aqueous phase was adjusted to pH=6 with 5% aqueous citric acid solution, extracted with ethyl acetate (3*20ml), the ethyl acetate phases were combined, dried over anhydrous magnesium sulfate, filtered, Purified by silica gel column chromatography (petroleum ether: ethyl acetate = 15:1) to obtain 60.31 mg of the title compound with a yield of 75.84%. Its hydrogen spectrum data are as follows: 1 H-NMR (400MHz, CDCl 3 ): δppm 3.731(s, 3H), 3.138(q, 1H), 3.092(q, 1H), 2.730(...
Embodiment 2
[0056] Example 2 3-mercapto-N-methoxy-2-methylpropionamide
[0057]
[0058] At room temperature (20°C to 30°C), the compound 3-acetylsulfanyl-N-methoxy-2-methylpropionamide (100.02 mg, 0.52 mmol) obtained in Preparation 2 above was dissolved in 10 ml of methanol , then add sodium bicarbonate (87.85mg, 1.05mmol), react at room temperature for 4 hours, then spin dry methanol, dissolve the residue in 15ml water, extract with petroleum ether (3*20ml), and then use 5% KHSO 4The aqueous solution was adjusted to pH = 6, extracted with ethyl acetate (3*20ml), the ethyl acetate phase was combined, dried over anhydrous magnesium sulfate, filtered, and subjected to silica gel column chromatography (petroleum ether: ethyl acetate = 15:1) Purification afforded 61.12 mg of the title compound with a yield of 78.33%. Its hydrogen spectrum data are as follows: 1 H-NMR (600MHz, CDCl 3 ): δppm8.372(s, 1H), 3.801(s, 3H), 2.879(m, 1H), 2.568(d, 1H), 2.290(t, 1H), 1.593(s, 1H), 1.272(d, 3H...
Embodiment 3
[0059] Embodiment 3 (S)-3-mercapto-N-methoxy-N,2-dimethylpropionamide
[0060]
[0061] At room temperature (20°C~30°C), the compound (S)-3-acetylsulfanyl-N-methoxy-N,2-dimethylpropanamide (100.08mg, 0.49 mmol) was dissolved in 10ml of methanol, then sodium bicarbonate (45.02mg, 0.54mmol) was added, reacted at room temperature for 8 hours, then the methanol was spin-dried, the residue was dissolved in 15ml of water, and extracted with petroleum ether (3*20ml) Impurities, then the aqueous phase was adjusted to pH=6 with 5% aqueous citric acid solution, extracted with ethyl acetate (3*20ml), the ethyl acetate phases were combined, dried over anhydrous magnesium sulfate, filtered, and the filtrate was spin-dried, 48.32 mg of the title compound were obtained, yield 60.76%. Its hydrogen spectrum data are as follows: 1 H-NMR (400MHz, CDCl 3 ): δppm 3.741(s, 3H), 3.211(s, 3H), 3.074(t, 1H), 2.884(q, 1H), 2.433(m, 1H), 1.532(s, 1H), 1.196(d, 3H ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com