Organic semiconductor material, preparation methods and uses thereof
An organic semiconductor and organic technology, applied in the preparation of organic semiconductor materials, organic semiconductor materials containing fluorene, can solve the mismatch between device spectral response and solar radiation spectrum, low carrier mobility of organic semiconductor devices, inorganic solar cells Low conversion efficiency and other issues, to achieve the effect of wide light absorption range, excellent solubility and charge transport performance, and improve carrier mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
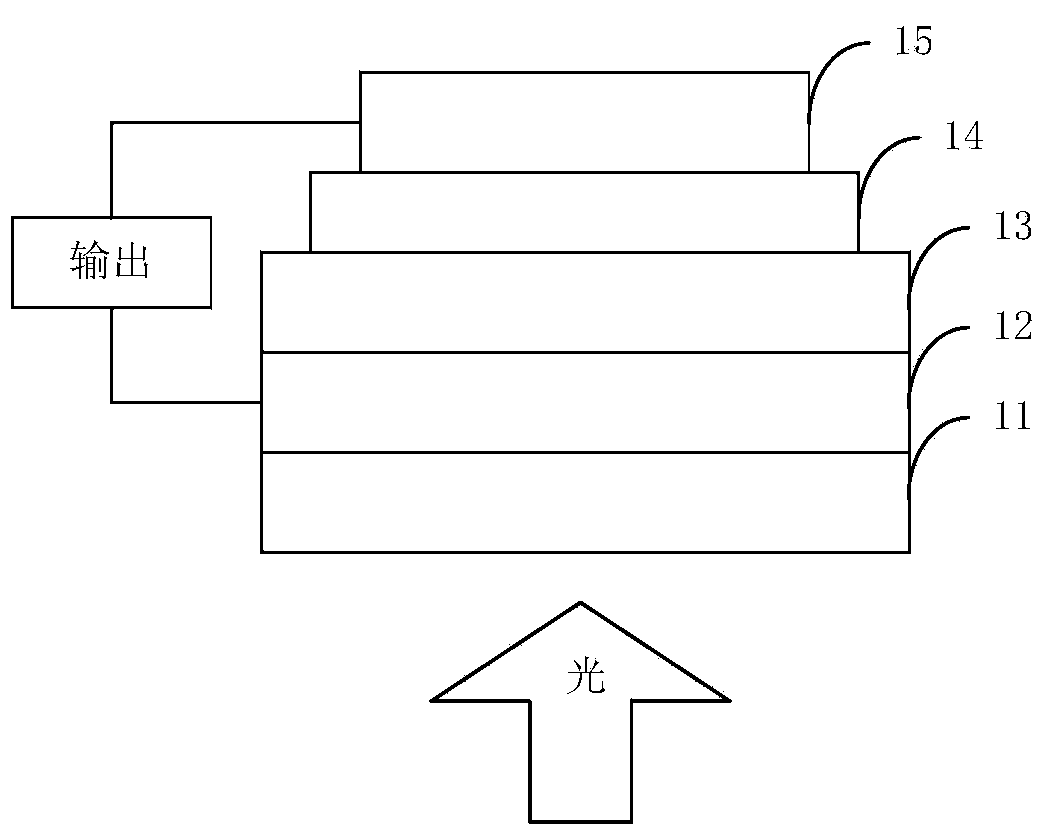
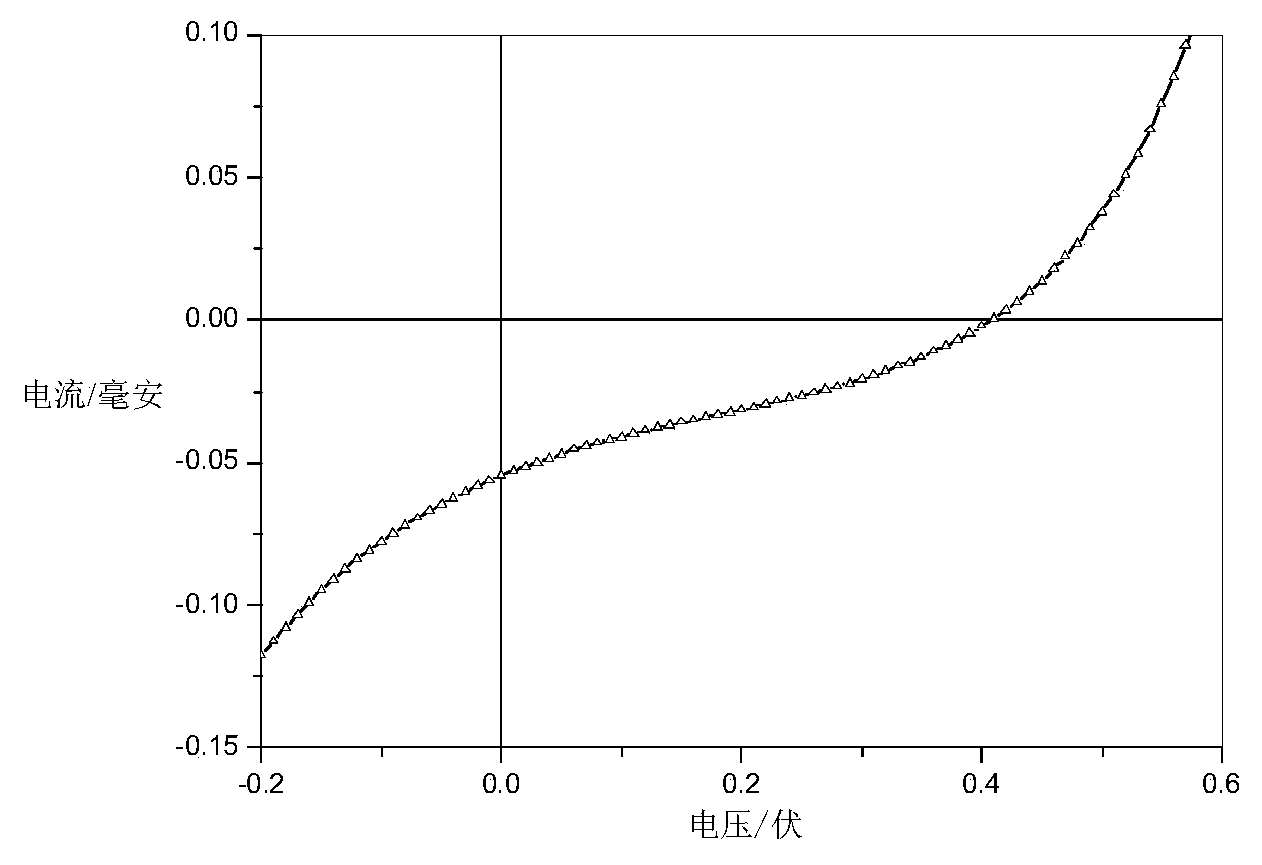
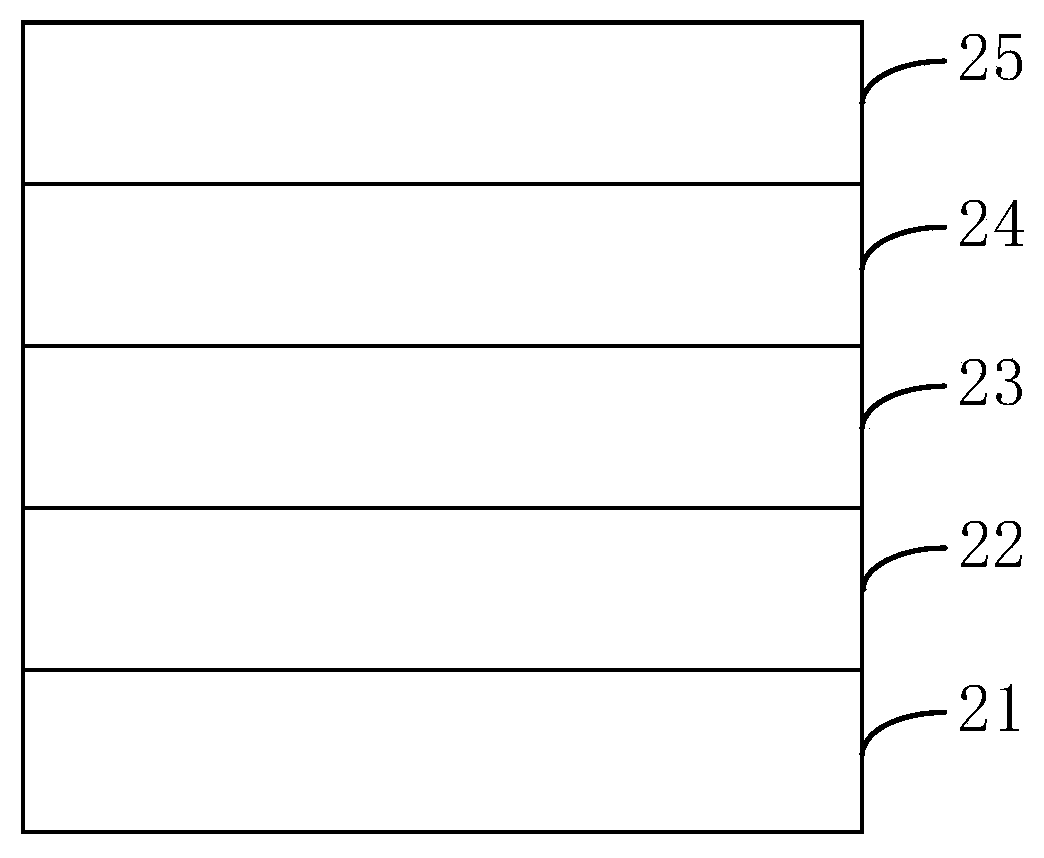
Image
Examples
preparation example Construction
[0036] The present invention also provides a method for preparing the above-mentioned organic semiconductor material, comprising the following steps:
[0037]Step S1. In an anhydrous and oxygen-free environment, mix 2,7-dibromo-9,9-dialkylfluorene and n-butyllithium (n-BuLi) at -70°C to -85°C at a molar ratio of 1.0: 2.0~1.0: Add 4.0 to the first solvent, then add 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (or bispina Alcohol diboron) (the molar amount is 2.0 to 4.0 times that of 2,7-dibromo-9,9-dialkylfluorene), warming up to 20-30°C, and reacting for 12-48 hours to obtain the product, namely 2 ,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolyl)-9,9-dialkylfluorene; wherein, the first solvent is tetrahydrofuran, diethyl ether , dichloromethane, chloroform or ethyl acetate, etc.; its reaction formula is as follows:
[0038]
[0039] In the formula, m is a natural number, and 1
[0040] Step S2. In an oxygen-free environment, 2,7-bis(4,4,5,5-...
Embodiment 1
[0054] Example 1 This example discloses organic semiconductor materials P1 and P2 with the following structures:
[0055]
[0056] The preparation steps of P1 and P2 are as follows:
[0057] Step 1. Preparation of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolyl)-9,9-dioctylfluorene:
[0058]
[0059] Set up an anhydrous and anaerobic reaction device, stirring and N 2 Under the protection of the three-necked bottle, add 9.0mmol of white 2,7-dibromo-9,9-dioctylfluorene, inject 150ml of refined tetrahydrofuran solvent with a syringe, and then slowly inject it with a syringe at -78°C 27.0mmol n-BuLi, stirred for 2 hours. After 2 hours, inject 30.6 mmol 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane with a syringe at -78°C, and raise the temperature to 22 °C, react for 14 hours.
[0060] After the reaction, add saturated NaCl aqueous solution, extract with chloroform, dry over anhydrous sodium sulfate, collect the filtrate after filtration and rotary evaporate the so...
Embodiment 2
[0067] Example 2 This example discloses organic semiconductor materials P3 and P4 with the following structures:
[0068]
[0069] The preparation steps of P3 and P4 are as follows:
[0070] Step 1. Preparation of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolyl)-9,9-dihexylfluorene:
[0071] Its preparation process refers to the step one difference in Example 1 in that:
[0072]In step S1, the temperature is -75°C, the temperature is raised to 20°C, and the reaction time is 48 hours; the molar ratio of 2,7-dibromo-9,9-dihexylfluorene to n-butyllithium is 1:2; the solvent is dichloromethane; The molar amount of 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane is 2,7-dibromo-9,9-dihexylfluorene mole 3 times the amount. .
[0073] Step 3, preparation of P3 and P4:
[0074]
[0075] Add 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan) base-9,9-dihexylfluorene 1mmol, 9, 10-dibromo-2,6-bis(2-octyldecyl)anthracene 0.8mmol (see Macromol.Chem.Phys.2006,207,1107-1115 of Kl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hole mobility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com