Application of 4-(benzofuran-5-yl)-2-phenzyl aminothiazole as bactericide
A technology of anilinothiazole and benzofuran, which is applied in the direction of fungicides, applications, and biocides, and can solve problems such as high toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] 4-(7-methoxy-2,2-dimethyl-2,3-dihydro-5-benzofuryl)-5-methyl-2-(2-methoxyanilino)thiazole to prepare
[0018]
[0019] 1) Preparation of 1-(7-methoxy-2,2-dimethyl-2,3-dihydro-5-benzofuryl)acetone
[0020] Under an ice-water bath, add 35.6 g of 7-methoxy-2,2-dimethyl-2,3-dihydrobenzofuran, 19.4 g of propionyl chloride, 30.7 g of aluminum trichloride and 150 mL of nitrobenzene, and mix After stirring for 5 h, TLC monitored the reaction to the end point. The reaction solution was poured into ice water, separated into layers, the solvent was evaporated, and recrystallized from ethanol / water to obtain 34.3 g of a light yellow solid with a yield of 73.1%. m.p.74.4~75.5℃.
[0021] 2) Preparation of 2-bromo-1-(7-methoxy-2,2-dimethyl-2,3-dihydro-5-benzofuryl)acetone
[0022] 11.0 g of 1-(7-methoxy-2,2-dimethyl-2,3-dihydro-5-benzofuryl)acetone and 100 mL of ethanol, heating, stirring, when the temperature rises to 60 ℃ , add 22.4 g of copper bromide in batches, heat pr...
Embodiment 2
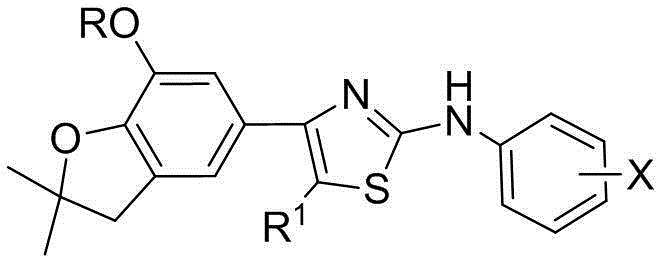
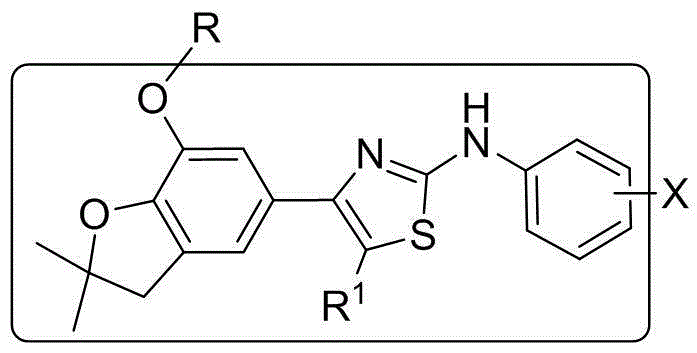
[0026] Preparation of 4-(benzofuran-5-yl)-2-anilinothiazole (Ⅰ)
[0027]
[0028] I
[0029] Wherein, R is selected from: C 1 ~C 2 Alkyl, C 3 ~C 4 Straight chain alkyl or branched chain alkyl; R 1 From: H, C 1 ~C 2 Alkyl, C 3 ~C 4 Straight-chain alkyl or branched-chain alkyl; X is selected from: 2-methoxy, 3-methoxy, 4-methoxy, 2-ethoxy, 3-ethoxy, 4-ethoxy, 2-trifluoromethoxy, 3-trifluoromethoxy, 4-trifluoromethoxy.
[0030] 4-(benzofuran-5-yl)-2-phenylaminothiazole was prepared according to the method of Example 1 or according to the Chinese invention patent [ZL 201010553848.9 and CN102603726A]].
Embodiment 3
[0032] Determination of the fungicidal activity of 4-(benzofuran-5-yl)-2-anilinothiazole
[0033] 1 Test purpose
[0034] The toxicity of the new compound to various pathogenic bacteria was determined indoors at the tested concentration, and its bactericidal activity was preliminarily evaluated.
[0035] 2 Test conditions
[0036] 2.1 Test target
[0037] Rapeseed Sclerotia sclerotiorum (Sclerotonia sclerotiorum), strains are stored in the refrigerator (4 ~ 8 ℃), 2-3 days before the test inoculated from the slant of the test tube into the petri dish, cultivated at a suitable temperature for the test. The experimental medium was potato agar (PDA).
[0038] 2.2 Culture conditions
[0039] The culture conditions of the tested target and the target after the test are temperature 25 ± 5°C, relative humidity 65 ± 5%
[0040] 2.3 Instruments and equipment
[0041] Beakers, pipettes, graduated cylinders, petri dishes, autoclaves, constant temperature biochemical incubators, etc....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com