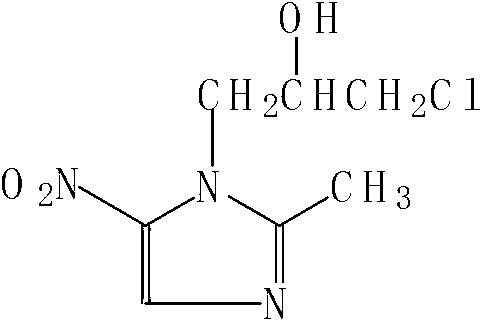
Ornidazole green synthetic method
A green synthesis, ornidazole technology, applied in chemical recycling, organic chemistry and other directions, can solve the problems of drug research and application limitations, inability to maintain normal production, unfavorable occupational health of employees, etc., to eliminate chemical pollution and save raw material costs. , The effect of saving solid waste treatment costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A green synthesis method for ornidazole, comprising the steps of: using 2-methyl-5-nitroimidazole and epichlorohydrin as raw materials, using ethyl acetate as a solvent, macroporous acidic polymer resin as a catalyst, epoxy Chloropropane is catalyzed to open the ring, and at the same time, it is alkylated and condensed with 2-methyl-5-nitroimidazolyl, and the crude product of ornidazole is obtained by one-step reaction, and the supernatant obtained after centrifugation is the ornidazole condensation liquid, which is separated After the supernatant, the mother liquor containing the catalyst is obtained, and after the mother liquor containing the catalyst is activated, the catalyst can be recycled; the ornidazole condensation liquid is distilled, the residue is decolorized, filtered and crystallized, and centrifuged again to obtain the required Ornidazole azole. The mass ratio of ethyl acetate, macroporous acidic polymer resin, epichlorohydrin and 2-methyl-5-nitroimidazol...
Embodiment 2
[0029]A green synthesis method for ornidazole, comprising the steps of: using 2-methyl-5-nitroimidazole and epichlorohydrin as raw materials, using ethyl acetate as a solvent, macroporous acidic polymer resin as a catalyst, epoxy Chloropropane is catalyzed to open the ring, and at the same time, it is alkylated and condensed with 2-methyl-5-nitroimidazolyl, and the crude product of ornidazole is obtained by one-step reaction, and the supernatant obtained after centrifugation is the ornidazole condensation liquid, which is separated After the supernatant, the mother liquor containing the catalyst is obtained, and after the mother liquor containing the catalyst is activated, the catalyst can be recycled; the ornidazole condensation liquid is distilled, the residue is decolorized, filtered and crystallized, and centrifuged again to obtain the required Ornidazole azole. The mass ratio of ethyl acetate, macroporous acidic polymer resin, epichlorohydrin and 2-methyl-5-nitroimidazole...
Embodiment 3
[0034] A green synthesis method for ornidazole, comprising the steps of: using 2-methyl-5-nitroimidazole and epichlorohydrin as raw materials, using ethyl acetate as a solvent, macroporous acidic polymer resin as a catalyst, epoxy Chloropropane is catalyzed to open the ring, and at the same time, it is alkylated and condensed with 2-methyl-5-nitroimidazolyl, and the crude product of ornidazole is obtained by one-step reaction, and the supernatant obtained after centrifugation is the ornidazole condensation liquid, which is separated After the supernatant, the mother liquor containing the catalyst is obtained, and after the mother liquor containing the catalyst is activated, the catalyst can be recycled; the ornidazole condensation liquid is distilled, the residue is decolorized, filtered and crystallized, and centrifuged again to obtain the required Ornidazole azole. The mass ratio of ethyl acetate, macroporous acidic polymer resin, epichlorohydrin and 2-methyl-5-nitroimidazol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com