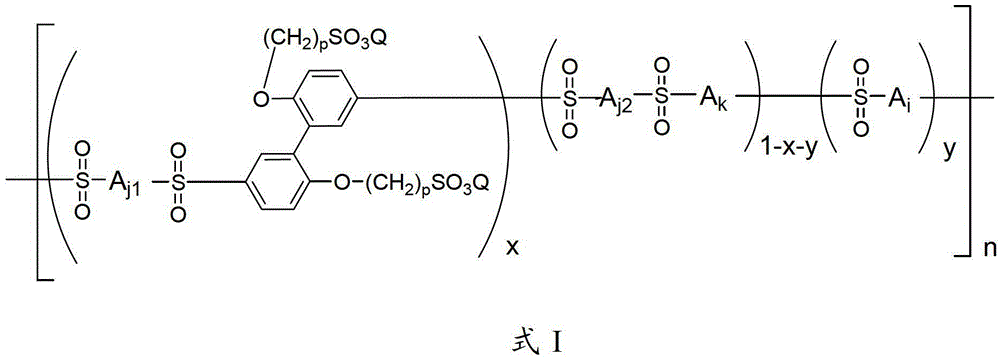
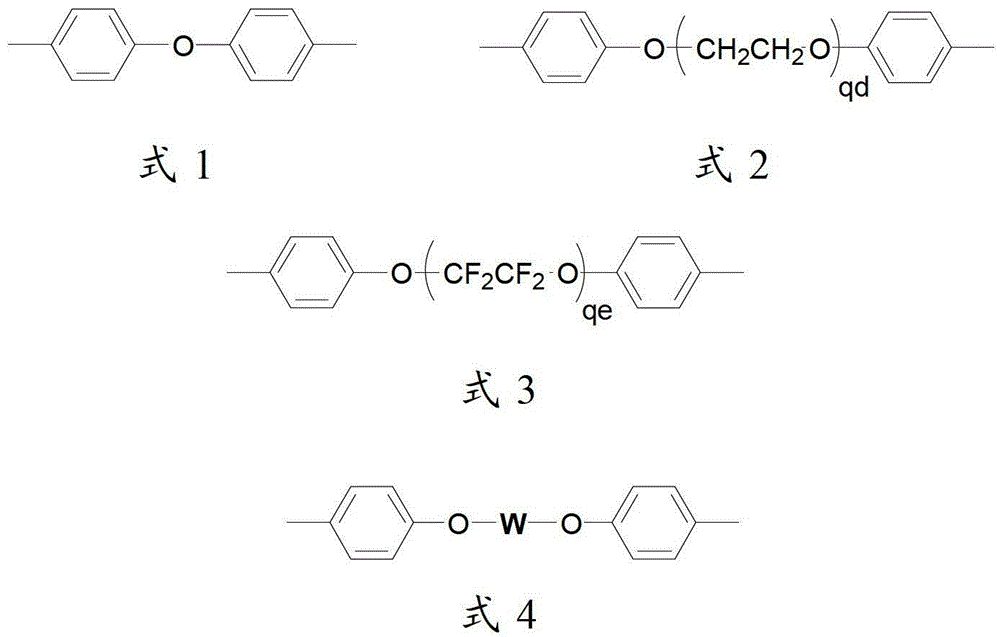
A kind of side chain sulfonated polysulfone and preparation method thereof
A sulfonation and side chain technology, which is applied to fuel cell parts, battery pack parts, electrical components, etc., can solve the problems of complex purification of polymer products and harsh polymerization conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] 0.01 mole of 2,2`-bis(3-sulfonated propoxy)biphenyl disodium, 1.00 mole of 4,4`-diphenyl ether disulfonic acid, 0.99 mole of 2,2`-dimethyl Add oxybiphenyl and 4 liters of Eaton's reagent into a nitrogen-gassed reaction bottle, stir and react at 80°C for 48 hours, pour the reaction liquid into a large amount of deionized water while hot to obtain a polymer precipitate, filter and wash repeatedly with deionized water until the filtrate is neutral, soak the obtained polymer with 0.1mol / L dilute hydrochloric acid at room temperature for 24 hours, then wash repeatedly with deionized water until the washing solution is neutral, and vacuum dry to obtain H + Polymers with side chains containing sulfonic acid groups in the form of polymers. In the infrared spectrum at 1310cm -1 with 1150cm -1 Strong stretching vibration peaks of sulfone groups appear on the left and right, indicating the occurrence of sulfone-based polymerization reaction; H NMR spectrum confirms that the stru...
Embodiment 2
[0057] 0.01 mole of 2,2'-bis(3-sulfonated propoxy)biphenyl disodium was synthesized according to the method of Example 1, 1.00 mole of 4,4'-diphenyl ether disulfonic acid, 0.99 mole Add 2,2`-dimethoxybiphenyl and 4 liters of Eaton's reagent into a nitrogen-gassed reaction flask, stir and react at 120°C for 24 hours, pour the reaction solution into a large amount of deionized water while it is hot to obtain a polymer precipitate, filter And wash repeatedly with deionized water until the filtrate is neutral, soak the obtained polymer with 0.1mol / L dilute hydrochloric acid at room temperature for 24 hours, then repeatedly wash with deionized water until the washing liquid is neutral, and vacuum dry to obtain H + Polymers with side chains containing sulfonic acid groups in the form of polymers. In the infrared spectrum at 1310cm -1 with 1150cm -1 Strong stretching vibration peaks of sulfone groups appear on the left and right, indicating the occurrence of sulfone-based polymeriz...
Embodiment 3
[0059] In turn, 0.01 moles of 2,2`-bis(3-sulfonated propoxy)biphenyl disodium were synthesized according to the method of Example 1, 1.00 moles of 4,4`-diphenyl ether disulfonic acid, 0.99 moles Add 2,2,-dimethoxybiphenyl and 4 liters of Eaton's reagent into a nitrogen-gassed reaction bottle, stir and react at 130°C for 10 hours, pour the reaction solution into a large amount of deionized water while it is hot to obtain a polymer precipitate, filter And wash repeatedly with deionized water until the filtrate is neutral, soak the obtained polymer with 0.1mol / L dilute hydrochloric acid at room temperature for 24 hours, then repeatedly wash with deionized water until the washing liquid is neutral, and vacuum dry to obtain H + Polymers with side chains containing sulfonic acid groups in the form of polymers. In the infrared spectrum at 1310cm -1 with 1150cm -1 Strong stretching vibration peaks of sulfone groups appear on the left and right, indicating the occurrence of sulfone-b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com