Preparation method of phenyl isothiocyanate
A technology of phenyl isothiocyanate and ethyl acetate, which is applied in the field of preparation of phenyl isothiocyanate, can solve the problems of serious environmental pollution, cumbersome post-processing, and difficult operation, and achieve simple equipment and short reaction time Short, easy-to-use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Weigh 1.23g of p-methoxyaniline, 2.28g of carbon disulfide and 0.56g of potassium hydroxide, place in a 25mL mechanochemical reaction tank, feed in parallel to another same mechanochemical reaction tank, and fix it after tightening the seal. On the swing arm of the German Leisch Mechanochemical Reactor (MM400). React for 40 minutes at an oscillation frequency of 30 Hz. After the reaction was completed, the reaction mixture of the two tanks was extracted with ethyl acetate / water, and the ethyl acetate phase was collected in combination, dried over anhydrous magnesium sulfate and filtered, and the filtrate was evaporated to dryness and separated by silica gel column chromatography (eluent: V Petroleum ether / V ethyl acetate=8:1) to obtain 1.55 g of pure p-methoxyphenyl isothiocyanate in the form of a white slightly yellow solid with a yield of 94%.
Embodiment 2-9
[0034] Adopt aniline or different substituted anilines to prepare phenyl isothiocyanate, experimental operation is the same as embodiment 1, and the results are shown in Table 1.
[0035] Table 1 is the phenyl isothiocyanate prepared by aniline or different substituted anilines as raw materials
[0036]
[0037] Table 1
[0038] The product nuclear magnetic resonance spectrogram of embodiment 1-6 (wherein resonant frequency is 300Hz, solvent used is CDCl 3 ) analysis results are as follows:
[0039] Product 1: p-methoxyphenylisothiocyanate
[0040] 1 HNMR: 7.16 (d, J = 8.6 Hz, 2H, ArH), 6.85 (d, J = 8.6 Hz, 2H, ArH), 3.80 (s, 3H, OCH 3 ); 13 CNMR: 158.6, 133.9 (NCS), 127.0, 123.5, 114.8, 55.6 (OCH 3 ).
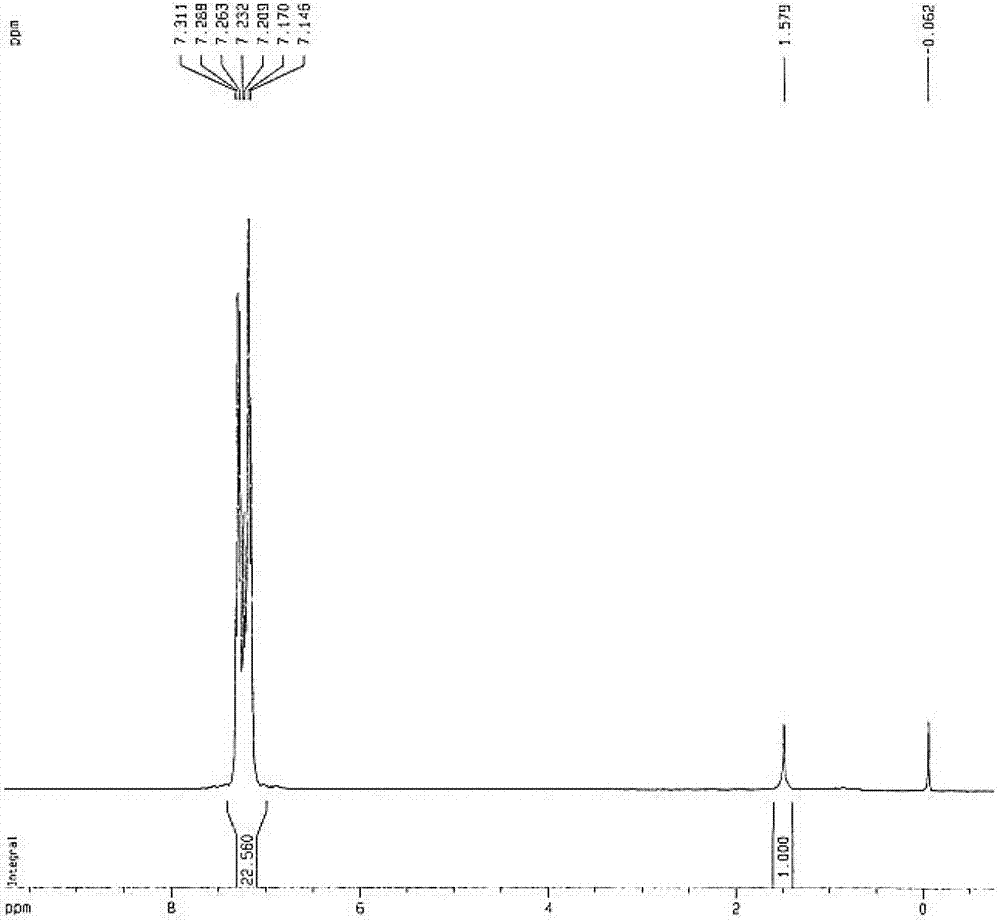
[0041] Product 2: phenylisothiocyanate
[0042] 1 HNMR: 7.15–7.31 (m, 5H, ArH); 13 CNMR: 134.6 (NCS), 130.3, 128.5, 126.3, 124.7.
[0043] Product 3: o-methylphenylisothiocyanate
[0044] 1 HNMR: 7.19 (brs, 4H, ArH), 2.39 (s, 3H, CH 3 ); 13 CNMR: 134.9, 133.8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com